Mastering MILD Procedure Billing and Coding: Essential Tips for Maximizing Reimbursement and Ensuring Compliance Discover expert strategies to navigate the complexities of MILD procedure billing and coding. Learn how to streamline the process, optimize reimbursement, and ensure compliance with payer guidelines and industry standards. The Vertos Medical Billing and Coding Guide for 2023 provides detailed information on how to bill and code for the MILD (Minimally Invasive Lumbar Decompression) procedure. The guide refers specifically to the coverage and billing policies of the Centers for Medicare & Medicaid Services (CMS) for this procedure. Here's a detailed and informative explanation of the key points from the guide:
0 Comments
Does this sound familiar? - BILLING CODING L5 DORSAL RAMUS AND S1, S2, S3 LATERAL BRANCH BLOCK When CPT code 64451 is performed from L5 to S3, it should be reported as a single unit of service regardless of the number of sacroiliac joints injected. Each sacroiliac joint should not be counted as a separate injection. This is because the injection is being performed at the sacral plexus, which is located near the sacroiliac joint but is a different structure.
This information is supported by the American Medical Association's CPT Assistant, which states that "when injection of the sacral plexus is performed at multiple levels (e.g., L4, L5, and S1), each level should be separately identified and reported with the -59 modifier appended to the additional levels beyond the first level." However, in the scenario you described, since the injection is being performed from L5 to S3, it should be reported as a single unit of service using code 64451 without any modifiers. It is important to review the payer's specific coding and billing guidelines to ensure compliance with their policies. The Centers for Medicare & Medicaid Services (CMS) has also published guidelines on the appropriate use of CPT code 64451. Source: American Medical Association. CPT Assistant. May 2013 Volume 23, Issue 5, Page 9. CMS National Correct Coding Initiative Policy Manual for Medicare Services, Chapter 11, Section H (available on the CMS website). CPT Codes to Report (based on Medical Necessity and Service(s) Performed: 63650 PERCUTANEOUS IMPLANTATION OF NEUROSTIMULATOR ELECTRODE ARRAY, EPIDURAL 63655 LAMINECTOMY FOR IMPLANTATION OF NEUROSTIMULATOR ELECTRODES, PLATE/PADDLE, EPIDURAL 63663 REVISION INCLUDING REPLACEMENT, WHEN PERFORMED, OF SPINAL NEUROSTIMULATOR ELECTRODE PERCUTANEOUS ARRAY(S), INCLUDING FLUOROSCOPY, WHEN PERFORMED 63664 REVISION INCLUDING REPLACEMENT, WHEN PERFORMED, OF SPINAL NEUROSTIMULATOR ELECTRODE PLATE/PADDLE(S) PLACED VIA LAMINOTOMY OR LAMINECTOMY, INCLUDING FLUOROSCOPY, WHEN PERFORMED 63685 INSERTION OR REPLACEMENT OF SPINAL NEUROSTIMULATOR PULSE GENERATOR OR RECEIVER, DIRECT OR INDUCTIVE COUPLING REMEMBER: to always review documentations and medical necessity when performing these services. According to CMS Utilization Guidelines: Utilization Guidelines (most commercial payers also follow this guidelines): 63650 - Two temporary spinal cord stimulator trials per anatomic spinal region (two per DOS) or (four units) per patient per lifetime (with exceptions allowed for technical limitations for the initial trials or for use of different modalities of stimulation, including new technology), in place of service office, ASC, out-patient hospital, or hospital. Since permanent neurostimulator arrays can also be placed percutaneously, code 63650 can be covered more often in place of service ASC, out-patient hospital, or hospital. 63655 - One permanent spinal cord stimulator per patient per lifetime and must be performed in an ASC, out-patient hospital or hospital. 63663 - Will not be reimbursed in the office setting since they are included in 63650. Remember: The imaging guidance is NON-BILLABLE! Common ICD-10 Codes Cross-over meeting Medical Necessity: M51.16 Intervertebral disc disorders with radiculopathy, lumbar region M51.17 Intervertebral disc disorders with radiculopathy, lumbosacral region M51.24 Other intervertebral disc displacement, thoracic region M51.25 Other intervertebral disc displacement, thoracolumbar region M51.26 Other intervertebral disc displacement, lumbar region M51.27 Other intervertebral disc displacement, lumbosacral region M54.11 Radiculopathy, occipito-atlanto-axial region M54.12 Radiculopathy, cervical region M54.13 Radiculopathy, cervicothoracic region M54.14 Radiculopathy, thoracic region M54.15 Radiculopathy, thoracolumbar region M54.16 Radiculopathy, lumbar region M54.17 Radiculopathy, lumbosacral region M54.18 Radiculopathy, sacral and sacrococcygeal region M96.1 Postlaminectomy syndrome, not elsewhere classified Medicare and Most PAYERS DO NOT reimburse for the Leads. So be careful not to report the L-Code not unless you know your payer will pay for it! Reference: https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleId=57792&ver=6 CPT CODE BOOK: 2021 and 2022 ICD-10 GUIDELINE: 2021 CPT is a Trademark and Owned by the American Medical Association SCS Vendors Useful Links: Boston Scientific Interventional Pain Management Products Medtronic Spinal Stimulation Systems NALU NeuroStimulation St. Jude Medical NeuroStimulation Systems (Abbott) Sharing to you all pain management billing codes we have been utilizing helping our pain practice offices, surgery centers and our physicians clients! Let me know if you have any questions, concerns or confusion on how to report these codes properly. We have been in this world of Pain Management Practice Operations and Documentation for more than 20 years! The truth is, Pain Management billing codes are not easy to utilize if you don't know how to use them. It is always useful that you understand your physicians documentations and their procedures. Most of these codes are unilateral. Most of these codes are based on utilization and frequency guidance. So make sure you know all your payers guidelines for clinical and reimbursement. Let me know if you need me! But here are you codes! Epidural Steroid Injections for Pain Management Billing Codes: 62321 INJECTION(S), OF DIAGNOSTIC OR THERAPEUTIC SUBSTANCE(S) (EG, ANESTHETIC, ANTISPASMODIC, OPIOID, STEROID, OTHER SOLUTION), NOT INCLUDING NEUROLYTIC SUBSTANCES, INCLUDING NEEDLE OR CATHETER PLACEMENT, INTERLAMINAR EPIDURAL OR SUBARACHNOID, CERVICAL OR THORACIC; WITH IMAGING GUIDANCE (IE, FLUOROSCOPY OR CT) 62323 INJECTION(S), OF DIAGNOSTIC OR THERAPEUTIC SUBSTANCE(S) (EG, ANESTHETIC, ANTISPASMODIC, OPIOID, STEROID, OTHER SOLUTION), NOT INCLUDING NEUROLYTIC SUBSTANCES, INCLUDING NEEDLE OR CATHETER PLACEMENT, INTERLAMINAR EPIDURAL OR SUBARACHNOID, LUMBAR OR SACRAL (CAUDAL); WITH IMAGING GUIDANCE (IE, FLUOROSCOPY OR CT) Epidural Steroid Injections for Pain Management Billing Codes: 64479 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; TRANSFORAMINAL EPIDURAL, WITH IMAGING GUIDANCE (FLUOROSCOPY OR CT), CERVICAL OR THORACIC, SINGLE LEVEL +64480 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; TRANSFORAMINAL EPIDURAL, WITH IMAGING GUIDANCE (FLUOROSCOPY OR CT), CERVICAL OR THORACIC, EACH ADDITIONAL LEVEL (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) 64483 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; TRANSFORAMINAL EPIDURAL, WITH IMAGING GUIDANCE (FLUOROSCOPY OR CT), LUMBAR OR SACRAL, SINGLE LEVEL +64484 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; TRANSFORAMINAL EPIDURAL, WITH IMAGING GUIDANCE (FLUOROSCOPY OR CT), LUMBAR OR SACRAL, EACH ADDITIONAL LEVEL (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) Facet Joint Injections and Medial Branch Blocks Pain Management Billing Codes 64490 INJECTION(S), DIAGNOSTIC OR THERAPEUTIC AGENT, PARAVERTEBRAL FACET (ZYGAPOPHYSEAL) JOINT (OR NERVES INNERVATING THAT JOINT) WITH IMAGE GUIDANCE (FLUOROSCOPY OR CT), CERVICAL OR THORACIC; SINGLE LEVEL +64491 INJECTION(S), DIAGNOSTIC OR THERAPEUTIC AGENT, PARAVERTEBRAL FACET (ZYGAPOPHYSEAL) JOINT (OR NERVES INNERVATING THAT JOINT) WITH IMAGE GUIDANCE (FLUOROSCOPY OR CT), CERVICAL OR THORACIC; SECOND LEVEL (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) +64492 INJECTION(S), DIAGNOSTIC OR THERAPEUTIC AGENT, PARAVERTEBRAL FACET (ZYGAPOPHYSEAL) JOINT (OR NERVES INNERVATING THAT JOINT) WITH IMAGE GUIDANCE (FLUOROSCOPY OR CT), CERVICAL OR THORACIC; THIRD AND ANY ADDITIONAL LEVEL(S) (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) 64493 INJECTION(S), DIAGNOSTIC OR THERAPEUTIC AGENT, PARAVERTEBRAL FACET (ZYGAPOPHYSEAL) JOINT (OR NERVES INNERVATING THAT JOINT) WITH IMAGE GUIDANCE (FLUOROSCOPY OR CT), LUMBAR OR SACRAL; SINGLE LEVEL +64494 INJECTION(S), DIAGNOSTIC OR THERAPEUTIC AGENT, PARAVERTEBRAL FACET (ZYGAPOPHYSEAL) JOINT (OR NERVES INNERVATING THAT JOINT) WITH IMAGE GUIDANCE (FLUOROSCOPY OR CT), LUMBAR OR SACRAL; SECOND LEVEL (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) +64495 INJECTION(S), DIAGNOSTIC OR THERAPEUTIC AGENT, PARAVERTEBRAL FACET (ZYGAPOPHYSEAL) JOINT (OR NERVES INNERVATING THAT JOINT) WITH IMAGE GUIDANCE (FLUOROSCOPY OR CT), LUMBAR OR SACRAL; THIRD AND ANY ADDITIONAL LEVEL(S) (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) Facet Joint Radiofrequency Neurotomy Pain Management Billing Codes 64633 DESTRUCTION BY NEUROLYTIC AGENT, PARAVERTEBRAL FACET JOINT NERVE(S), WITH IMAGING GUIDANCE (FLUOROSCOPY OR CT); CERVICAL OR THORACIC, SINGLE FACET JOINT +64634 DESTRUCTION BY NEUROLYTIC AGENT, PARAVERTEBRAL FACET JOINT NERVE(S), WITH IMAGING GUIDANCE (FLUOROSCOPY OR CT); CERVICAL OR THORACIC, EACH ADDITIONAL FACET JOINT (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) 64635 DESTRUCTION BY NEUROLYTIC AGENT, PARAVERTEBRAL FACET JOINT NERVE(S), WITH IMAGING GUIDANCE (FLUOROSCOPY OR CT); LUMBAR OR SACRAL, SINGLE FACET JOINT +64636 DESTRUCTION BY NEUROLYTIC AGENT, PARAVERTEBRAL FACET JOINT NERVE(S), WITH IMAGING GUIDANCE (FLUOROSCOPY OR CT); LUMBAR OR SACRAL, EACH ADDITIONAL FACET JOINT (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) Peripheral Nerve Blocks Pain Management Billing Codes 64400 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; TRIGEMINAL NERVE, EACH BRANCH (IE, OPHTHALMIC, MAXILLARY, MANDIBULAR) 64405 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; GREATER OCCIPITAL NERVE 64415 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; BRACHIAL PLEXUS 64416 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; BRACHIAL PLEXUS, CONTINUOUS INFUSION BY CATHETER (INCLUDING CATHETER PLACEMENT) 64417 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; AXILLARY NERVE 64418 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; SUPRASCAPULAR NERVE 64420 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; INTERCOSTAL NERVE, SINGLE LEVEL +64421 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; INTERCOSTAL NERVE, EACH ADDITIONAL LEVEL (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) 64425 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; ILIOINGUINAL, ILIOHYPOGASTRIC NERVES 64430 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; PUDENDAL NERVE 64445 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; SCIATIC NERVE 64446 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; SCIATIC NERVE, CONTINUOUS INFUSION BY CATHETER (INCLUDING CATHETER PLACEMENT) 64447 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; FEMORAL NERVE 64448 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; FEMORAL NERVE, CONTINUOUS INFUSION BY CATHETER (INCLUDING CATHETER PLACEMENT) 64449 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; LUMBAR PLEXUS, POSTERIOR APPROACH, CONTINUOUS INFUSION BY CATHETER (INCLUDING CATHETER PLACEMENT) 64450 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; OTHER PERIPHERAL NERVE OR BRANCH 64454 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; GENICULAR NERVE BRANCHES, INCLUDING IMAGING GUIDANCE, WHEN PERFORMED 64455 INJECTION(S), ANESTHETIC AGENT(S) AND/OR STEROID; PLANTAR COMMON DIGITAL NERVE(S) (EG, MORTON'S NEUROMA) 64624 DESTRUCTION BY NEUROLYTIC AGENT, GENICULAR NERVE BRANCHES INCLUDING IMAGING GUIDANCE, WHEN PERFORMED 64620 DESTRUCTION BY NEUROLYTIC AGENT, INTERCOSTAL NERVE 64640 DESTRUCTION BY NEUROLYTIC AGENT; OTHER PERIPHERAL NERVE OR BRANCH 64999 UNLISTED PROCEDURE, NERVOUS SYSTEM Related Imaging Pain Management Billing Codes: 76881 ULTRASOUND, COMPLETE JOINT (IE, JOINT SPACE AND PERI-ARTICULAR SOFT-TISSUE STRUCTURES), REAL-TIME WITH IMAGE DOCUMENTATION 76882 ULTRASOUND, LIMITED, JOINT OR OTHER NONVASCULAR EXTREMITY STRUCTURE(S) (EG, JOINT SPACE, PERI-ARTICULAR TENDON[S], MUSCLE[S], NERVE[S], OTHER SOFT-TISSUE STRUCTURE[S], OR SOFT-TISSUE MASS[ES]), REAL-TIME WITH IMAGE DOCUMENTATION 76942 ULTRASONIC GUIDANCE FOR NEEDLE PLACEMENT (EG, BIOPSY, ASPIRATION, INJECTION, LOCALIZATION DEVICE), IMAGING SUPERVISION AND INTERPRETATION 76999 UNLISTED ULTRASOUND PROCEDURE (EG, DIAGNOSTIC, INTERVENTIONAL) 97032 APPLICATION OF A MODALITY TO 1 OR MORE AREAS; ELECTRICAL STIMULATION (MANUAL), EACH 15 MINUTES 97139 UNLISTED THERAPEUTIC PROCEDURE (SPECIFY) G0282 ELECTRICAL STIMULATION, (UNATTENDED), TO ONE OR MORE AREAS, FOR WOUND CARE OTHER THAN DESCRIBED IN G0281 G0283 ELECTRICAL STIMULATION (UNATTENDED), TO ONE OR MORE AREAS FOR INDICATION(S) OTHER THAN WOUND CARE, AS PART OF A THERAPY PLAN OF CARE Pain Management Billing Codes for Spinal Cord Stimulators for Chronic Pain 63650 PERCUTANEOUS IMPLANTATION OF NEUROSTIMULATOR ELECTRODE ARRAY, EPIDURAL 63655 LAMINECTOMY FOR IMPLANTATION OF NEUROSTIMULATOR ELECTRODES, PLATE/PADDLE, EPIDURAL 63663 REVISION INCLUDING REPLACEMENT, WHEN PERFORMED, OF SPINAL NEUROSTIMULATOR ELECTRODE PERCUTANEOUS ARRAY(S), INCLUDING FLUOROSCOPY, WHEN PERFORMED 63664 REVISION INCLUDING REPLACEMENT, WHEN PERFORMED, OF SPINAL NEUROSTIMULATOR ELECTRODE PLATE/PADDLE(S) PLACED VIA LAMINOTOMY OR LAMINECTOMY, INCLUDING FLUOROSCOPY, WHEN PERFORMED 63685 INSERTION OR REPLACEMENT OF SPINAL NEUROSTIMULATOR PULSE GENERATOR OR RECEIVER, DIRECT OR INDUCTIVE COUPLING Pay Attention to Medicare's Utilization Guidelines. This is also being utilized by most payers! 63650 - Two temporary spinal cord stimulator trials per anatomic spinal region (two per DOS) or (four units) per patient per lifetime (with exceptions allowed for technical limitations for the initial trials or for use of different modalities of stimulation, including new technology), in place of service office, ASC, out-patient hospital, or hospital. Since permanent neurostimulator arrays can also be placed percutaneously, code 63650 can be covered more often in place of service ASC, out-patient hospital, or hospital. 63655 - One permanent spinal cord stimulator per patient per lifetime and must be performed in an ASC, out-patient hospital or hospital. 63663 - Will not be reimbursed in the office setting since they are included in 63650. Pain Management Billing Codes for Kyphoplasty and Vertebroplasty 22510 PERCUTANEOUS VERTEBROPLASTY (BONE BIOPSY INCLUDED WHEN PERFORMED), 1 VERTEBRAL BODY, UNILATERAL OR BILATERAL INJECTION, INCLUSIVE OF ALL IMAGING GUIDANCE; CERVICOTHORACIC 22511 PERCUTANEOUS VERTEBROPLASTY (BONE BIOPSY INCLUDED WHEN PERFORMED), 1 VERTEBRAL BODY, UNILATERAL OR BILATERAL INJECTION, INCLUSIVE OF ALL IMAGING GUIDANCE; LUMBOSACRAL +22512 PERCUTANEOUS VERTEBROPLASTY (BONE BIOPSY INCLUDED WHEN PERFORMED), 1 VERTEBRAL BODY, UNILATERAL OR BILATERAL INJECTION, INCLUSIVE OF ALL IMAGING GUIDANCE; EACH ADDITIONAL CERVICOTHORACIC OR LUMBOSACRAL VERTEBRAL BODY (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) 22513 PERCUTANEOUS VERTEBRAL AUGMENTATION, INCLUDING CAVITY CREATION (FRACTURE REDUCTION AND BONE BIOPSY INCLUDED WHEN PERFORMED) USING MECHANICAL DEVICE (EG, KYPHOPLASTY), 1 VERTEBRAL BODY, UNILATERAL OR BILATERAL CANNULATION, INCLUSIVE OF ALL IMAGING GUIDANCE; THORACIC 22514 PERCUTANEOUS VERTEBRAL AUGMENTATION, INCLUDING CAVITY CREATION (FRACTURE REDUCTION AND BONE BIOPSY INCLUDED WHEN PERFORMED) USING MECHANICAL DEVICE (EG, KYPHOPLASTY), 1 VERTEBRAL BODY, UNILATERAL OR BILATERAL CANNULATION, INCLUSIVE OF ALL IMAGING GUIDANCE; LUMBAR +22515 PERCUTANEOUS VERTEBRAL AUGMENTATION, INCLUDING CAVITY CREATION (FRACTURE REDUCTION AND BONE BIOPSY INCLUDED WHEN PERFORMED) USING MECHANICAL DEVICE (EG, KYPHOPLASTY), 1 VERTEBRAL BODY, UNILATERAL OR BILATERAL CANNULATION, INCLUSIVE OF ALL IMAGING GUIDANCE; EACH ADDITIONAL THORACIC OR LUMBAR VERTEBRAL BODY (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) Botox and ChemoDenervation Pain Management Billing Codes 64612 CHEMODENERVATION OF MUSCLE(S); MUSCLE(S) INNERVATED BY FACIAL NERVE, UNILATERAL (EG, FOR BLEPHAROSPASM, HEMIFACIAL SPASM)
64615 CHEMODENERVATION OF MUSCLE(S); MUSCLE(S) INNERVATED BY FACIAL, TRIGEMINAL, CERVICAL SPINAL AND ACCESSORY NERVES, BILATERAL (EG, FOR CHRONIC MIGRAINE) 64616 CHEMODENERVATION OF MUSCLE(S); NECK MUSCLE(S), EXCLUDING MUSCLES OF THE LARYNX, UNILATERAL (EG, FOR CERVICAL DYSTONIA, SPASMODIC TORTICOLLIS) 64617 CHEMODENERVATION OF MUSCLE(S); LARYNX, UNILATERAL, PERCUTANEOUS (EG, FOR SPASMODIC DYSPHONIA), INCLUDES GUIDANCE BY NEEDLE ELECTROMYOGRAPHY, WHEN PERFORMED 64640 DESTRUCTION BY NEUROLYTIC AGENT; OTHER PERIPHERAL NERVE OR BRANCH 64642 CHEMODENERVATION OF ONE EXTREMITY; 1-4 MUSCLE(S) 64643 CHEMODENERVATION OF ONE EXTREMITY; EACH ADDITIONAL EXTREMITY, 1-4 MUSCLE(S) (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) 64644 CHEMODENERVATION OF ONE EXTREMITY; 5 OR MORE MUSCLES 64645 CHEMODENERVATION OF ONE EXTREMITY; EACH ADDITIONAL EXTREMITY, 5 OR MORE MUSCLES (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) 64646 CHEMODENERVATION OF TRUNK MUSCLE(S); 1-5 MUSCLE(S) 64647 CHEMODENERVATION OF TRUNK MUSCLE(S); 6 OR MORE MUSCLES HCPCS Pain Management Billing Codes for Botox and Chemodenervation J0585 INJECTION, ONABOTULINUMTOXINA, 1 UNIT J0586 INJECTION, ABOBOTULINUMTOXINA, 5 UNITS J0587 INJECTION, RIMABOTULINUMTOXINB, 100 UNITS J0588 INJECTION, INCOBOTULINUMTOXIN A, 1 UNIT Effective November 1, 2021 United Healthcare will require you to request Prior Authorization for the following services for Interventional Pain Management. This is effective November 1, 2021. Notice that mostly are for your Cervical & Thoracic regions. Notice that it is also required for your Genicular Nerve Block (but NOT for the Genicular Nerve RFA) and SI (but for the RFA). Codes that require Prior Authorization from UHC:United Healthcare Pain Management Injection Requiring Prior Authorization Effective November 1, 2021 CPT Code Description 62292 INJECTION PX CHEMONUCLEOLYSIS 1/MLT LUMBAR 64620 DSTRJ NEUROLYTIC AGENT INTERCOSTAL NERVE G0260 INJ PROC SI JNT;ANES STEROID&TX AGT&ARTHROGRPH 62320 NJX DX/THER SBST INTRLMNR CRV/THRC W/O IMG GDN 62322 NJX DX/THER SBST INTRLMNR LMBR/SAC W/O IMG GDN 62324 NJX DX/THER SBST INTRLMNR CRV/THRC W/O IMG GDN 62325 NJX DX/THER SBST INTRLMNR CRV/THRC W/IMG GDN 62326 NJX DX/THER SBST INTRLMNR LMBR/SAC W/O IMG GDN 62327 NJX DX/THER SBST INTRLMNR LMBR/SAC W/IMG GDN 62350 IMPLANT SPINAL CANAL CATHETER 62351 IMPLANT SPINAL CANAL CATHETER 62360 INSERT SPINE INFUSION DEVICE 62361 IMPLTJ/RPLCMT FS NON-PRGRBL PUMP 64451 INJECTION AA&/STRD NERVES NRVTG SI JOINT W/IMG 64454 INJECTION AA&/STRD GENICULAR NRV BRANCHES W/IMG 64480 NJX AA&/STRD TFRML EPI CERVICAL/THORACIC EA ADDL 64484 NJX AA&/STRD TFRML EPI LUMBAR/SACRAL EA ADDL 64491 NJX DX/THER AGT PVRT FACET JT CRV/THRC 2ND LEVEL 64492 NJX DX/THER AGT PVRT FACET JT CRV/THRC 3+ LEVEL 64494 NJX DX/THER AGT PVRT FACET JT LMBR/SAC 2ND LEVEL 64495 NJX DX/THER AGT PVRT FACET JT LMBR/SAC 3+ LEVEL 64520 INJECTION ANES LMBR/THRC PARAVERTBRL SYMPATHETIC 64634 DSTR NROLYTC AGNT PARVERTEB FCT ADDL CRVCL/THORA 64636 DSTR NROLYTC AGNT PARVERTEB FCT ADDL LMBR/SACRAL 64640 DSTRJ NEUROLYTIC AGENT OTHER PERIPHERAL NERVE Read more about this update here: https://www.uhcprovider.com/content/dam/provider/docs/public/resources/network-bulletin/pain-inject-management-nb-appendix-auwww.uhcprovider.com/content/dam/provider/docs/public/resources/network-bulletin/pain-inject-management-nb-appendix-august-2021.pdfgust-2021.pdf Today is October 8, 2021. Have you not noticed your claims are being rejected by your practice management clearinghouse as "Invalid ICD-10 Code"? ICD-10 Pain Management Code M54.5 Deleted Effective October 1, 2021! Yes?! Well, it is because the ICD-10 code M54.5 for Low Back Pain is now deleted effective all date of service from October 1, 2021! So make sure you are aware of this and correct your claims by choosing the billable and appropriate ICD-10 code that will describe your deleted code M54.5. Belo are some potential code replacements that you can use beginning October 1, 2021
New Codes you can potentially use: Remember that when changes like this happens, this is not just for CMS Medicare claims but it applies to all commercial payers (at least the big insurance payers!). Read more about this updates and change by clicking here https://www.cms.gov/medicare/icd-10/2022-icd-10-cm Billing for the Genicular Nerve Branches RFA have been a struggle since it was not too clear to us on how we should be billing for this service. The good news is, we have a new code for this effective January 1, 2020. New CPT 2020 Changes. New Pain Management 2020 Codes. When your physician is performing an RFA on Genicular nerves, use code 64624 (Destruction by neurolytic agent of genicular nerve branches). Take note of the word "branches". These changes are explained as follows: Destruction by Neurolytic Agent (eg, Chemical, Thermal, Electrical or Radiofrequency), Chemodenervation on the Somatic Nerves CPT CODE 64624 Destruction by neurolytic agent, genicular nerve branches including imaging guidance, when performed (Do not report 64624 in conjunction with 64454 - Injection(s), anesthetic agent(s) and/or steroid; genicular nerve branches, including imaging guidance, when performed Pay attention to this, the CPT 64624 requires the destruction of each of the following genicular nerve branches: (make sure your Provider had documented this!)
If a neurolytic agent for the purposes of destruction is not applied to all of these nerve branches, you can report CPT 64624 but you MUST append the MODIFIER 52: 64624-52 What is Modifier 52? Modifier 52 is usually used for reduced services. It may occur under certain circumstances that a service or procedure is partially reduced or eliminated at the physician’s discretion. There can be several reasons behind the physician’s decision. In this situation, service provided can be identified by its usual procedure number and the addition of the modifier 52, which indicates that the service was reduced. Understanding the 3 Genicular Nerve Branches of 64624 Image Source: https://ainsworthinstitute.com/genicular-neurotomy/ What is the CPT code for Knee Genicular Nerve Branches Block or Injection?Understanding the 3 Genicular Nerve Branches of 64454 Source: https://ainsworthinstitute.com/genicular-neurotomy/ When your Physician is Blocking the Knee Genicular Nerves - here's your code: (pay attention with the imaging! it is included!). CPT 64454 - Injection(s), anesthetic agent(s) and/or steroid; genicular nerve branches; (make sure your Provider had documented this!)
If all 3 of these genicular nerve branches are not injected, report 64454 with Modifier; 64454-52 What is Modifier 52? Modifier 52 is usually used for reduced services. It may occur under certain circumstances that a service or procedure is partially reduced or eliminated at the physician’s discretion. There can be several reasons behind the physician’s decision. In this situation, service provided can be identified by its usual procedure number and the addition of the modifier 52, which indicates that the service was reduced. Article Source: CPT Assistant December 2019 page 8 Destruction by Neurolytic Agent (Genicular Injection; Radiofrequency Neurotomy Sacroiliac Joint) For Current Procedural Terminology Got additional questions or concerns? call us today! Billing Tip: Always make sure you understand and you know the Medical, Clinical, Utilization and Reimbursement Policy of your Payers. Read other blog posts:HOW TO BILL FOR 20553 WITH 76942 ULTRASOUNDPAIN MANAGEMENT NERVE BLOCKS January 1, 2020 - we now have a new Pain Management Code CPT 64625 - SI Ablation
Description of CPT Code 64625 Radiofrequency ablation, nerves innervating the sacroiliac joint, with imaging guidance (Fluoroscopic or Computed Tomography). Keypoints to REMEMBER!
Need help? Contact our office today! This blog post provides a comprehensive guide on how to correctly bill for MILD (Minimally-Invasive Lumbar Decompression) procedures in the healthcare industry. With clear instructions and helpful tips, the post aims to assist healthcare providers in navigating the billing process, avoiding common errors, and maximizing reimbursements. Whether you are a physician, coder, or biller, this informative post from GoHealthcare Practice Solutions LLC can serve as a valuable resource for billing MILD procedures accurately and efficiently. Some of my Pain Practice Offices are still confused on how to bill for MILD Procedure. As we all remember, there was no assigned CPT Code for this procedure, we used to report the unlisted code. In this Blog, I will describe the billing and coding for this procedure using the Vertos Device (www.vertosmed.com First, let's describe what is MILD? MILD stands for MINIMALLY INVASIVE LUMBAR DECOMPRESSION. HOW TO BILL FOR MILD - MINIMALLY INVASIVE LUMBAR DECOMPRESSION CPT 0275T is a Category III Code assigned for this procedure. 0275T - Percutaneous laminotomy/laminectomy (interlaminar approach) for decompression of neural elements, (with or without ligamentous resection, discectomy, facetectomy, and/or foraminotomy), any method, under indirect image guidance (eg. fluoroscopic, CT), single or multiple levels, unilateral or bilateral; lumbar. (For percutaneous decompression of the nuleus pulposus of intervertebral disc utilizing needle based technique, use 62287) So how do you report and bill for this MILD Procedure? Billing on HCFA 1500 form? Physician Claim:
The following information has to appear and included in your claim submission:
My additional recommendation (especially for Medicare beneficiaries): Enter the "referring physician" on Box 17 the same as the "rendering physician" on Box 24J since the assessment and treatment plan is that from the "Rendering Physician". 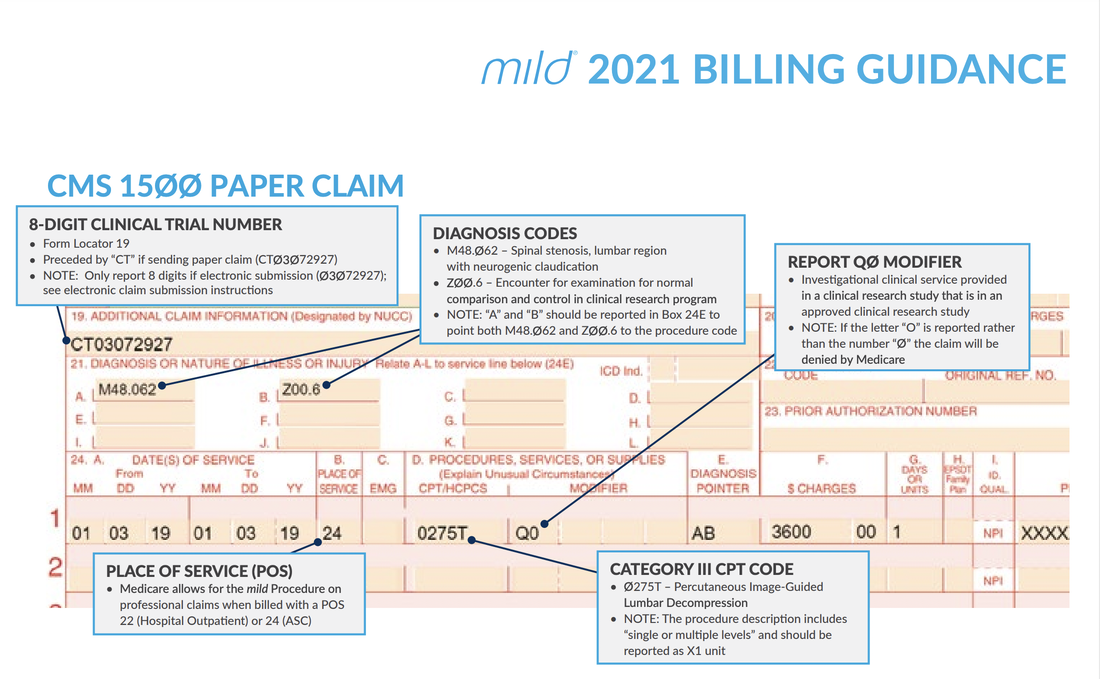 Image/Guidance Source: www.vertosmed.com Image/Guidance Source: www.vertosmed.com 2022 GUIDANCE HOW TO BILL FOR MILD - MINIMALLY INVASIVE LUMBAR DECOMPRESSION BILLING GUIDANCE for the Procedure (NCTØ3Ø72927) - SOURCE VERTOS (SEE ATTACHMENT BELOW)
Video Source is owned by: VERTOS MED - www.vertosmed.com Do you need additional help with billing?READER'S QUESTION: Does Medicare Cover Radiofrequency Ablation for Pain Management in New York? Here's the Coverage Information from Medicare Part BIndications:
General Procedure Requirements:
Diagnostic Facet Joint Injections
Therapeutic Injections
Thermal Medial Branch Radiofrequency Neurotomy (includes RF and microwave technologies):
Limitations of Coverage: A maximum of five (5) facet joint injection sessions inclusive of medial branch blocks, intraarticular injections, facet cyst rupture and RF ablations may be performed per year in the cervical/thoracic spine and five (5) in the lumbar spine.
Let's describe the CPT codes 64633-64636 CPT CODE 64633 DESTRUCTION BY NEUROLYTIC AGENT, PARAVERTEBRAL FACET JOINT NERVE(S), WITH IMAGING GUIDANCE (FLUOROSCOPY OR CT); CERVICAL OR THORACIC, SINGLE FACET JOINT CPT CODE +64634 DESTRUCTION BY NEUROLYTIC AGENT, PARAVERTEBRAL FACET JOINT NERVE(S), WITH IMAGING GUIDANCE (FLUOROSCOPY OR CT); CERVICAL OR THORACIC, EACH ADDITIONAL FACET JOINT (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) CPT CODE 64635 DESTRUCTION BY NEUROLYTIC AGENT, PARAVERTEBRAL FACET JOINT NERVE(S), WITH IMAGING GUIDANCE (FLUOROSCOPY OR CT); LUMBAR OR SACRAL, SINGLE FACET JOINT CPT CODE +64636 DESTRUCTION BY NEUROLYTIC AGENT, PARAVERTEBRAL FACET JOINT NERVE(S), WITH IMAGING GUIDANCE (FLUOROSCOPY OR CT); LUMBAR OR SACRAL, EACH ADDITIONAL FACET JOINT (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) Source reference: LCD ID L35936 Facet Joint Injections, Medial Branch Blocks, and Facet Joint Radiofrequency Neurotomy NGS Medicare Part B National Government Services, Inc. MAC - Part A 06101 - MAC A J - 06 Illinois National Government Services, Inc. MAC - Part B 06102 - MAC B J - 06 Illinois National Government Services, Inc. MAC - Part A 06201 - MAC A J - 06 Minnesota National Government Services, Inc. MAC - Part B 06202 - MAC B J - 06 Minnesota National Government Services, Inc. MAC - Part A 06301 - MAC A J - 06 Wisconsin National Government Services, Inc. MAC - Part B 06302 - MAC B J - 06 Wisconsin National Government Services, Inc. A and B and HHH MAC 13101 - MAC A J - K Connecticut National Government Services, Inc. A and B and HHH MAC 13102 - MAC B J - K Connecticut National Government Services, Inc. A and B and HHH MAC 13201 - MAC A J - K New York - Entire State National Government Services, Inc. A and B and HHH MAC 13202 - MAC B J - K New York - Downstate National Government Services, Inc. A and B and HHH MAC 13282 - MAC B J - K New York - Upstate National Government Services, Inc. A and B and HHH MAC 13292 - MAC B J - K New York - Queens National Government Services, Inc. A and B and HHH MAC 14111 - MAC A J - K Maine National Government Services, Inc. A and B and HHH MAC 14112 - MAC B J - K Maine National Government Services, Inc. A and B and HHH MAC 14211 - MAC A J - K Massachusetts National Government Services, Inc. A and B and HHH MAC 14212 - MAC B J - K Massachusetts National Government Services, Inc. A and B and HHH MAC 14311 - MAC A J - K New Hampshire National Government Services, Inc. A and B and HHH MAC 14312 - MAC B J - K New Hampshire National Government Services, Inc. A and B and HHH MAC 14411 - MAC A J - K Rhode Island National Government Services, Inc. A and B and HHH MAC 14412 - MAC B J - K Rhode Island National Government Services, Inc. A and B and HHH MAC 14511 - MAC A J - K Vermont National Government Services, Inc. A and B and HHH MAC 14512 - MAC B J - K Vermont How to Document radiofrequency ablation (RFA) of nerves CPT (64635, +64636) Radiofrequency ablation (RFA), also called radiofrequency neurotomy is an interventional pain management procedure that involves heating a part of a pain-transmitting nerve with a radiofrequency needle to create a heat lesion. Some of our pain physicians offices are asking the question - How to Document radiofrequency ablation (RFA) of nerves CPT (64635, +64636. What is the proper way of reporting this kind of procedure on the medical record when performed? Here's a guidance from CPT Assistant Article published on May 2020, quotes: Question: When performing radiofrequency ablation (RFA) of nerves (64635, 64636), is it necessary that the operative report documents the specific facet joints at which the RFA with imaging occurred as well as the nerves treated or denervated? Answer: Yes, RFA procedures should clearly state which nerves were ablated and which joints were treated. Codes 64635, Destruction by neurolytic agent, paravertebral facet joint nerve(s), with imaging guidance (fluoroscopy or CT); lumbar or sacral, single facet joint, and 64636, Destruction by neurolytic agent, paravertebral facet joint nerve(s), with imaging guidance (fluoroscopy or CT); lumbar or sacral, each additional facet joint (List separately in addition to code for primary procedure), are reported for each joint treated, not each nerve treated. Stating the specific nerve and the level it innervates eliminates confusion and ensures accurate reporting. Reference: CPT Assistant Published on May 2020 How to Bill for Knee Genicular Nerve Branches RFA or Ablation, Destruction Billing for the Genicular Nerve Branches RFA have been a struggle since it was not too clear to us on how we should be billing for this service. The good news is, we have a new code for this effective January 1, 2020. New CPT 2020 Changes. New Pain Management 2020 Codes. When your physician is performing an RFA on Genicular nerves, use code 64624 (Destruction by neurolytic agent of genicular nerve branches). Take note of the word "branches". These changes are explained as follows: Destruction by Neurolytic Agent (eg, Chemical, Thermal, Electrical or Radiofrequency), Chemodenervation on the Somatic Nerves CPT CODE 64624 Destruction by neurolytic agent, genicular nerve branches including imaging guidance, when performed (Do not report 64624 in conjunction with 64454 - Injection(s), anesthetic agent(s) and/or steroid; genicular nerve branches, including imaging guidance, when performed Pay attention to this, the CPT 64624 requires the destruction of each of the following genicular nerve branches: (make sure your Provider had documented this!)
If a neurolytic agent for the purposes of destruction is not applied to all of these nerve branches, you can report CPT 64624 but you MUST append the MODIFIER 52: 64624-52 What is Modifier 52? Modifier 52 is usually used for reduced services. It may occur under certain circumstances that a service or procedure is partially reduced or eliminated at the physician’s discretion. There can be several reasons behind the physician’s decision. In this situation, service provided can be identified by its usual procedure number and the addition of the modifier 52, which indicates that the service was reduced. Understanding the 3 Genicular Nerve Branches of 64624What is the CPT code for Knee Genicular Nerve Branches Block or Injection? Understanding the 3 Genicular Nerve Branches of 64454When your Physician is Blocking the Knee Genicular Nerves - here's your code: (pay attention with the imaging! it is included!). CPT 64454 - Injection(s), anesthetic agent(s) and/or steroid; genicular nerve branches; (make sure your Provider had documented this!)
If all 3 of these genicular nerve branches are not injected, report 64454 with Modifier; 64454-52 What is Modifier 52? Modifier 52 is usually used for reduced services. It may occur under certain circumstances that a service or procedure is partially reduced or eliminated at the physician’s discretion. There can be several reasons behind the physician’s decision. In this situation, service provided can be identified by its usual procedure number and the addition of the modifier 52, which indicates that the service was reduced. Article Source: CPT Assistant December 2019 page 8 Destruction by Neurolytic Agent (Genicular Injection; Radiofrequency Neurotomy Sacroiliac Joint) For Current Procedural Terminology Got additional questions or concerns? call us today! Billing Tip: Always make sure you understand and you know the Medical, Clinical, Utilization and Reimbursement Policy of your Payers. Read other blog posts:When your Pain Physician performed a Peripheral Nerve Blocks (unilateral) at the Dorsal Ramus Nerve levels L5, S1, S2 and S3, we would always look on CPT Codes 64450 (Injection, anesthetic agent; other peripheral nerve or branch) for the S1, S2 and S3. Here's the good news! Effective January 1, 2020, we now have a more specific code instead of using the "other peripheral" nerve block. Our 2020 Pain Management New Code is:
64451, Injection(s), anesthetic agent(s) and/or steroid; nerves innervating the sacroiliac joint, with image guidance (ie, fluoroscopy or CT computed tomography), should be reported once for this procedure. The fluoroscopic guidance should not be separately reported as it is included in the work described with code 64451. Modifier 58 Staged or Related Procedure or Service During Postoperative Period by Same Physician  Guideline: The same physician planned, at time of original surgery/procedure, a return trip to operating or procedure room within 10 or 90 day post op days WHEN IT IS APPROPRIATE:
Physicians in same specialty, same group are to bill and are reimbursed as a single physician Key to Remember! Use modifier 78 (not 58!) for treatment problems unplanned requiring return trip to operating room If hardware removed in unplanned surgery return for a complication, (e.g. infection of the wound site or rejection of the hardware itself), modifier 78 appropriate It is NOT APPROPRIATE WHEN:
References:
CMS Medicare Website Coding Books Payers Websites Still confused with Coding and reporting medial and lateral branch nerve blocks and understanding Pain Management procedures? Coding Billing for Medial and Lateral Nerve Blocks. According to the AMA, the code series for medial branch blocks and the facet joint injections are the same (i.e., CPT series 64490-64495), with reporting based on the number of facet joints injected, not the number of nerves injected. For example: If three (3) medial branch nerves are injected only two (2) facet joint injection codes would be reported despite the fact that three nerves were injected, since each facet joint is connected to two medial nerves. The lateral branch nerve is a peripheral nerve and would be reported with CPT code 64450, Injection, anesthetic agent; other peripheral nerve or branch, when a lateral branch nerve block is performed. Please note: CPT code 64450 should only be reported per nerve or branch and not per injection. CPT code 76942, Ultrasonic guidance for needle placement (e.g., biopsy, aspiration, injection, localization device), imaging supervision and interpretation, would be additionally reported when utilizing ultrasound guidance for certain nerve block procedures when it is not inherent in the primary procedure code. The Different kinds or types of NERVE BLOCKS and what are they targeting: NERVE BLOCKS: Brachial plexus block, elbow block, wrist block BODY AREAS: Shoulder, arm, hand, elbow, wrist) NERVE BLOCKS: Cervical epidural, thoracic epidural, lumbar epidural block BODY AREAS: Neck, back NERVE BLOCKS: Cervical plexus block, cervical paravertebral block BODY AREAS: Shoulder, upper neck NERVE BLOCKS: Maxillary nerve block BODY AREAS: Upper jaw NERVE BLOCKS: Ophthalmic nerve block BODY AREAS: Eyelids, scalp NERVE BLOCKS: Sphenopalatine nerve block BODY AREAS: Nose, palate NERVE BLOCKS: Subarachnoid block, Celiac plexus block BODY AREAS: Abdomen, pelvis NERVE BLOCKS: Supraorbital nerve block BODY AREAS: Forehead NERVE BLOCKS: Trigeminal nerve block BODY AREAS: Face CPT 64490, 64493, 64495, 64633 - Billing and Coding for Facet Nerve Block and Nerve Ablation RFA CPT CODE 64490 PARAVERTEBRAL FACET JOINT BILLING AND CODING WITH IMAGING GUIDANCEInjection(s), diagnostic or therapeutic agent, paravertebral facet (zygapophyseal) joint (or nerves innervating that joint) with image guidance (fluoroscopy or CT), cervical or thoracic; single level 64491 ----------- second level 64492 ----------- third and any additional level(s) level CPT CODE 64493Injection(s), diagnostic or therapeutic agent, paravertebral facet (zygapophyseal) joint (or nerves innervating that joint) with image guidance (fluoroscopy or CT), lumbar or sacral; single level 64494 ----------- second level 64495 ----------- third and any additional level(s) level FACET JOINT BILLING AND CODING WITH ULTRA-SOUND 0213T Injection(s), diagnostic or therapeutic agent, paravertebral facet (zygapophyseal) joint (or nerves innervating that joint) with ultrasound guidance, cervical or thoracic; single level ....................................................................+ 0214T second level ....................................................................+ 0215T third and any additional level(s) 0216TInjection(s), diagnostic or therapeutic agent, paravertebral facet (zygapophyseal) joint (or nerves innervating that joint) with ultrasound guidance, lumbar or sacral; single level ..................................................................+ 0217T second level ..................................................................+ 0218T third and any additional level(s) Billing and Coding for Radiofrequency Facet denervationCPT CODE 64633 Destruction by neurolytic agent, paravertebral facet joint nerve(s), with imaging guidance (fluoroscopy or CT); cervical or thoracic, single facet joint' +64634 cervical or thoracic, each additional facet joint (List separately in addition to code for primary procedure) CPT CODE 64635 Destruction by neurolytic agent, paravertebral facet joint nerve(s), with imaging guidance (fluoroscopy or CT); lumbar or sacral, single facet joint; +64636 lumbar or sacral, each additional facet joint (List separately in addition to code for primary procedure) Other Searched Keywords: Billing and Coding for RFA of Facet Joint Nerves Billing and Coding for Facet Joint AblationKey Points for CPT 64490, 64493, 64495, 64633 - Billing and Coding for Facet Nerve Block and Nerve Ablation RFA These codes are unilateral
 Let's look at these questions and answers: #1 Question "What are the appropriate code assignments when a patient receives 3 separate nerve blocks into the same lateral branch nerve? Would it be appropriate to report 3 units of this service?" The right CPT code 64450, Injection, anesthetic agent; other peripheral nerve or branch, would be appropriately reported only once in this case since all 3 nerve blocks were administered to the same nerve or branch. #2 Question "We are getting conflicting information regarding coding medial and lateral branch blocks S1, S2, and S3, Medial 64493, 64494, Lateral 64493, and 64494. Our Pain Center wants to use facet injection for the medial branch block and other peripheral nerve for the lateral branch block. Are we correct in reporting lateral branch nerve block(s) to the peripheral nerve CPT code?" Yes, you are correct. The lateral branches of the dorsal sacral nerve plexus are considered peripheral nerves. Therefore, for the four lateral branch block injections at S1, S2, S3, and S4, report 4 units of CPT code 64450, Injection, anesthetic agent; other peripheral nerve or branch. Report multiple units of the injection for the four lateral branch block injections performed, modifier 59 would not be appended in this case. # 3 Question "A patient was seen at our facility and underwent a left-sided L5 and S1, S2, S3, and S4 lateral branch nerve block for diagnostic purpose with C-arm fluoroscopy. What are the correct codes for a lateral nerve block?" So OK, ... based on the operative report a medial branch nerve block was performed at the L5 and a lateral branch nerve block was performed at the S1, S2, S3 and S4 Therefore, it would be appropriate to report CPT code 64493, Injection(s), diagnostic or therapeutic agent, paravertebral facet (zygapohphyseal) joint (or nerves innervating that joint) with image guidance (fluoroscopy or CT), lumbar or sacral, single level, for the L5 medial branch block. For the 4 lateral branch block injections at S1, S2, S3, and S4, report 4 units of CPT code 64450, Injection, anesthetic agent; other peripheral nerve or branch. From AMA's CPT Assistant: February 2011 page 4 (In September 2011 questions relating to this article were discussed.) Revisions made to certain pain medicine procedures in the CPT 2011 codebook include new procedure codes, and guidelines were created in the Nervous System section to clarify the reporting of these services. The following code sets are affected: • Introduction/Injection of Anesthetic Agent (Nerve Block), Diagnostic or Therapeutic (64400-64530) • Neurostimulators (Peripheral Nerve) (64550-64595) • Destruction by Neurolytic Agent (eg, Chemical, Thermal, Electrical or Radiofrequency) (64600-64681) Introduction/Injection of Anesthetic Agent (Nerve Block), Diagnostic or Therapeutic Revised Codes The following codes were revised for 2011: 64479Injection(s), anesthetic agent and/or steroid, transforaminal epidural, with imaging guidance (fluoroscopy or CT); cervical or thoracic, single level 64480Injection(s), anesthetic agent and/or steroid, transforaminal epidural, with imaging guidance (fluoroscopy or CT); cervical or thoracic, each additional level (List separately in addition to code for primary procedure) 64483Injection(s), anesthetic agent and/or steroid, transforaminal epidural, with imaging guidance (fluoroscopy or CT); lumbar or sacral, single level 64484Injection(s), anesthetic agent and/or steroid, transforaminal epidural, with imaging guidance (fluoroscopy or CT); lumbar or sacral, each additional level (List separately in addition to code for primary procedure) What exactly is "transforaminal epidural injection"? codes 64479-64484. TFE describe injections that enter the epidural space through the intervetebral foramen. This technique differs from interlaminar / translaminar epidural injection technique (62321-62327) and the paravertebral facet joint nerve injection technique (64490-64495). Since the vertebral artery (in the cervical spine), radiculomedullary arteries, as well as the spinal cord are in close proximity to the nerve root, this procedure involves a much higher risk with more work than a translaminar epidural injection. If ultrasound is used to guide the transforaminal injections, a code from the category III code set should be used instead of a code from the 64479-64484 code series. Therefore, parenthetical notes instruct users to report Category III codes 0228T, 0229T, 0230T, and 0231T for ultrasound-guided transforaminal epidural procedures. Additionally ultrasound guidance procedure code 76942, Ultrasound guidance for needle placement (eg biopsy, aspiration, injection, localization device), imaging supervision and interpretation, has been revised to clarify that it may not be used as guidance for 64479-64495 injections. Coding Tip Codes 64479-64484 are inherently unilateral procedures. When these procedures are performed bilaterally, they should be appended with modifier 50 or with a HCPCS Level II modifier "RT" or "LT" depending upon payer requirements. Paravertebral Spinal Nerves and Branches New Guidelines The paravertebral facet joint is the site of interaction between the vertebral bone above and below, and can be a source of pain. Injections can be made either into the joint, or at each of the nerves that supply the joint (ie, the medial nerve branches). To coordinate with the revision of codes 64479-64484, new parenthetical notes in the Paravertebral Spinal Nerves and Branches section of the CPT codebook direct users to the appropriate code to identify paravertebral facet joint injections when performed with imaging guidance. When performing a paravertebral facet injection into the T12-L1 joint, or at the nerves innervating that joint, code 64490 is reported. Fluoroscopy and CT imaging guidance and any injection of contrast are inclusive components of codes 64490- 64495. Imaging guidance and localization are required for the performance of paravertebral facet joint injections, as described by codes 64490-64495. If imaging guidance is not used, code 20552, Injection(s); single or multiple trigger point(s), 1 or 2 muscle(s), or code 20553 , Injection(s); single or multiple trigger point(s), 3 or more muscle(s), should be reported instead of a code from the 64490-64495 code series. If ultrasound guidance is used, it is appropriate to report Category III codes 0213T-0218T. Coding Tip Paravertebral facet injection codes 64490-64495 and 0213T-0218T are unilateral. When performed bilaterally, they may be appended with modifier 50 or a HCPCS Level II modifier "RT" or "LT" depending on the requirements of the payer. Neurostimulators (Peripheral Nerve) New Codes Code 64573 was deleted and the following four new codes were added for 2011: 64566Posterior tibial neurostimulation, percutaneous needle electrode, single treatment, includes programming 64568Incision for implantation of cranial nerve (eg, vagus nerve) neurostimulator electrode array and pulse generator 64569Revision or replacement of cranial nerve (eg, vagus nerve) neurostimulator electrode array, including connection to existing pulse generator 64570Removal of cranial nerve (eg, vagus nerve) neurostimulator electrode array and pulse generator Code 64566 is reported for a treatment of voiding dysfunction (eg, urge incontinence), posterior tibial nerve stimulation. Code 64566 was created to describe a minimally invasive procedure that includes both the needle insertion through the skin adjacent to the tibial nerve, as well as the placement of an electrode on the surface of the skin. The treatment consists of a series of sessions involving insertions of a percutaneous needle electrode, with intermittent neuromodulation for approximately 30 minutes while the needle electrode remains in place. The neurostimulator includes a lead set with surface electrodes and a needle electrode, which produces an adjustable electrical pulse that travels to the sacral nerve plexus via the tibial nerve. The sacral nerve plexus then regulates the bladder and the pelvic floor functionality. Code 64566 would be reported once for each neurostimulation treatment session. References:
2017 / 2018 Coding Books (CPT is a Trademark and Owned by the American Medical Association) AMA's CPT Assistant Archives CMS Medicare Website (LMN, NCD/LCD, Manuals) Commercial Payers Guidelines Definition: Increased Procedural Service requiring work substantially greater than typically required.
The RIGHT WAY:
When the modifier 22 is used, two separate documents will be required to support the claim:
Important Information for Billing and Documentation Based on Medicare's Guideline of which most payers does follow Medicare's Guideline. So pay attention on this: If you append a 22 modifier to a procedure you will receive an Additional Documentation Request (ADR) letter requesting medical records to support the use of the 22 Modifier. It is important that both the operative report and a separate concise statement on why it was beyond the normal difficulty be returned with a copy of the ADR letter. Failure to submit the statement and documentation in a timely fashion will result in processing of the claim with the fee schedule rate for the same surgery submitted without the 22 modifier. Documentation Tips: When developing a separate statement avoid using a generalized statement. Comments like "patient was obese" or "surgery took longer than usual" or "multiple adhesions" lack specific details which identify why the procedure was beyond the normal difficulties that could be encountered with the procedure. Further, it is important that your operative note supports the statement on why the surgical procedure was beyond the ordinary range of difficulty. Unassigned Claim For unassigned claims, an increase in the limiting charge is allowed only when a charge above the fee schedule amount is justified. Reference CMS Manual Instruction: The CMS Internet-Only Manual (IOM) Publication 100-04, Chapter 12 , Section 20.4.6 shows the requirements for using this modifier. Billing and Coding for Orthopedic Spinal Fusion Let's begin with some terminology to remember;
Understanding the Posterior Lumbar Interbody Spinal Fusion 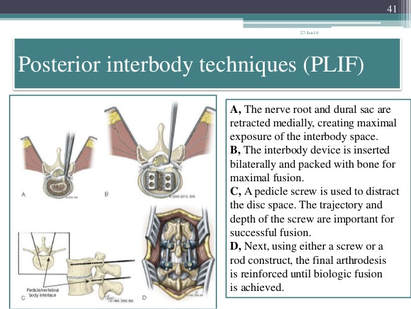 Techniques:
Image Source: https://www.slideshare.net/drpraveenktripathi/lumbar-interbody-fusion-indications-techniques-and-complications
Your CPT® Codes for PLIF and TLIF Spinal Fusion Coding: CPT Code 22630, +22632 22630 Arthrodesis, posterior interbody technique, including laminectomy and/or discectomy to prepare interspace (other than for decompression), single interspace; lumbar +22632 Each Additional interspace (list separately in addition to code for primary procedure code) Here's what occurs when 22630 is performed: The provider performs an arthrodesis, also known as spinal fusion, in the lumbar spine, or lower back, to permanently join two vertebrae, the interlocking bones of the spine. He excises the lamina and disk material and applies bone graft between the disks to fuse them. The procedure helps to alleviate persistent pain caused by various spinal conditions, including herniated intervertebral disks, stenosis, or spinal injuries. Then, in 2012 Code 22633 was introduced to to represent the combination of 22630 and 22612 Arthrodesis, posterior or posterolateral technique, single level; lumbar (with lateral transverse technique, when performed) at the same level. The Anterior Interbody Fusion Approach
Videos to watch for Procedure PLIF and TLIF Your CPT® Codes for ALIF, DLIF and OLIF Spinal Fusion Coding: CPT Code 22558, +22585 22558 Arthrodesis, anterior interbody technique, including minimal discectomy to prepare interspace (other than for decompression); lumbar Remember: (For arthrodesis using pre-sacral interbody technique, see 22586, 0195T) +22585 Arthrodesis, anterior interbody technique, including minimal discectomy to prepare interspace (other than for decompression); each additional interspace (List separately in addition to code for primary procedure) Remember: (Use 22585 in conjunction with 22554, 22556, 22558) (Do not report 22585 in conjunction with 63075, even if performed by a separate individual. To report anterior cervical discectomy and interbody fusion at the same level during the same session, use 22552) Here's what occurs when 22558 is being performed: The provider performs arthrodesis, also known as spinal fusion, in the lower back, to permanently join two vertebrae, the interlocking bones of the spine, to alleviate persistent pain caused by a herniated, or bulging, disk, or other spinal condition. He makes an incision in the abdomen to access the spine and remove disk material. Instrumentation may be required to stabilize the Spinal Fusion POSTERIOR INSTRUMENTATION: Add-Code +22840 Posterior non-segmental instrumentation (eg, Harrington rod technique, pedicle fixation across 1 interspace, atlantoaxial transarticular screw fixation, sublaminar wiring at C1, facet screw fixation) (List separately in addition to code for primary procedure) (Use 22840 in conjunction with 22100-22102, 22110-22114, 22206, 22207, 22210-22214, 22220-22224, 22310-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040-63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081, 63085, 63087, 63090, 63101, 63102, 63170-63290, 63300-63307) Add-On Code +22841 Internal spinal fixation by wiring of spinous processes (List separately in addition to code for primary procedure) Add-On Code +22842 Posterior segmental instrumentation (eg, pedicle fixation, dual rods with multiple hooks and sublaminar wires); 3 to 6 vertebral segments (List separately in addition to code for primary procedure) Use 22842 in conjunction with 22100- 22102, 22110- 22114, 22206, 22207, 22210- 22214, 22220-22224, 22305-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040-63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081, 63085, 63087, 63090, 63101, 63102, 63170-63290, 63300- 63307)Text has been updated Add-On Code +22843 7 to 12 vertebral segments (List separately in addition to code for primary procedure) (Use 22843 in conjunction with 22100- 22102, 22110-22114, 22206, 22207, 22210- 22214, 22220-22224, 22305-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040-63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081,63085, 63087, 63090, 63101, 63102, 63170-63290,63300- 63307)Text has been updated Add-On Code +22844 13 or more vertebral segments (List separately in addition to code for primary procedure) (Use 22844 in conjunction with 22100- 22102, 22110-22114, 22206, 22207, 22210- 22214, 22220-22224, 22305-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040-63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081, 63085, 63087, 63090, 63101, 63102, 63170-63290, 63300- 63307) ANTERIOR INSTRUMENTATION Add-On Code +22845 Anterior instrumentation; 2 to 3 vertebral segments (List separately in addition to code for primary procedure) (Use 22845 in conjunction with 22100- 22102, 22110-22114, 22206, 22207, 22210- 22214, 22220-22224, 22305-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040-63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081,63085, 63087, 63090, 63101, 63102, 63170-63290,63300- 63307)Text has been updated Add-On Code +22846 4 to 7 vertebral segments (List separately in addition to code for primary procedure) Use 22846 in conjunction with 22100- 22102, 22110-22114, 22206, 22207, 22210- 22214, 22220-22224, 22305-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040-63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081, 63085, 63087, 63090, 63101, 63102, 63170-63290, 63300- 63307)Text has been updated Add-On Code +22847 8 or more vertebral segments (List separately in addition to code for primary procedure) (Use 22847 in conjunction with 22100-22102, 22110-22114, 22206, 22207, 22210-22214, 22220-22224, 22305-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040- 63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081, 63085, 63087, 63090, 63101, 63102, 63170-63290,63300-63307)Text has been updated Add-On Code +22848 Pelvic fixation (attachment of caudal end of instrumentation to pelvic bony structures) other than sacrum (List separately in addition to code for primary procedure) (Use 22848 in conjunction with 22100- 22102, 22110-22114, 22206, 22207, 22210- 22214, 22220-22224, 22305-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040-63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081, 63085, 63087, 63090, 63101, 63102, 63170-63290, 63300- 63307)  Co-Surgeon Modifier 62 may not be appended with your Instrumentation Codes! Spinal Prosthetic Devices may also be required to be reported CPT Code 22853 22853 Insertion of interbody biomechanical device(s) (eg, synthetic cage, mesh) with integral anterior instrumentation for device anchoring (eg, screws, flanges), when performed, to intervertebral disc space in conjunction with interbody arthrodesis, each interspace (List separately in addition to code for primary procedure) Notes: (Use 22853 in conjunction with 22100-22102, 22110-22114, 22206, 22207, 22210-22214, 22220-22224, 22310-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040-63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081, 63085, 63087, 63090, 63101, 63102, 63170-63290, 63300-63307) (Report 22853 for each treated intervertebral disc space) Code +22853 is one of several new codes within the spine section for the insertion of biomechanical devices that replace deleted code +22851 (Application of intervertebral biomechanical device[s] ...). The new add-on codes are more specific regarding the type and location of the biomechanical devices. CPT® guidelines direct you to report +22853 for each treated intervertebral disc space. Report +22853 in addition to the definitive procedure(s) since it is an add-on code. Do not append modifier 62 (Two surgeons) to 22853. The provider inserts a metallic cage or mesh device between two vertebrae and may use screws or flanges to attach it to the front part of the vertebrae; the device maintains the disc space, provides spinal stability, and yet preserves some range of motion, which helps relieve persistent pain caused by a herniated, or bulging, disk or other spinal condition. The provider performs this procedure during a spinal interbody arthrodesis procedure, which is fusion, or permanent joining, of vertebrae over the joint space. Remember! Code +22853 is an add–on code and must be reported with an appropriate primary procedure, such as 22548–22586 (Anterior or anterolateral approach technique arthrodesis procedures on the spine [vertebral column]), but there are many other codes that can be reported as a primary code. Report one unit of this code for each interspace treated, not for the number of devices inserted. For example, if the provider inserts two cages into a single interspace, you report this code only once. If the provider inserts a device at two separate interspaces, e.g., between C3–4 and C5–6, then you would report this code twice. This code is for the application of a device to expand or maintain an intervertebral disc space. For a similar procedure to cover a defect created by removal of a vertebral body, report 22854 (Insertion of intervertebral biomechanical device(s) [e.g., synthetic cage, mesh] with integral anterior instrumentation for device anchoring [e.g., screws, flanges], when performed, to vertebral corpectomy[ies] [vertebral body resection, partial or complete] defect, in conjunction with interbody arthrodesis, each contiguous defect [List separately in addition to code for primary procedure]). For insertion of a similar device to treat an intervertebral disc space or vertebral body removal defect but without interbody fusion (arthrodesis), report 22859 (Insertion of intervertebral biomechanical device[s] [e.g., synthetic cage, mesh, methylmethacrylate] to intervertebral disc space or vertebral body defect without interbody arthrodesis, each contiguous defect [List separately in addition to code for primary procedure]). Report Bone Grafting if allowable, CPT Code 20930 20930 Allograft, morselized, or placement of osteopromotive material, for spine surgery only (List separately in addition to code for primary procedure) Notes: (Use 20930 in conjunction with 22319, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812) Here's what occurs when 20930 is being performed; The provider applies small pieces of donor or synthetic bone graft material during a spinal surgery to encourage bone growth during the healing period. Coding Tip! Code 20930 is an add on code and used for specified spinal procedures only. Check with your payer to determine if 20930 can be billed separately or if the application of the bone graft material is included in the code for the primary surgical procedure. Do not append modifier 62 to bone graft codes 20900-20938. (For spinal surgery bone graft[s] see codes 20930-20938) Check with your payer if you can separately report this code; +20931 Allograft, structural, for spine surgery only (List separately in addition to code for primary procedure) Notes: (Use 20931 in conjunction with 22319, 22532-22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812) A provider uses a structural allograft, a type of donor bone, to fill in bony defects as she performs a spinal surgery procedure. Coding Tips: Code 20931 is an add on code describing application of structural allograft to spinal defects and must be reported with an allowable primary spinal procedure code. Report 20930, Allograft, morselized, or placement of osteopromotive material, for spine surgery only, together with 20931 only in the case of a human donor who is a different person from the recipient. You should never append modifier 50, Bilateral procedure, to 20931. The CMS Physician Fee Schedule Database includes a 9 indictor in the BILAT SURG column for this code. According to further CMS instructions, a 9 indicator in this column means that the concept of a bilateral surgery with spinal grafting does not apply. +20936 Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process, or laminar fragments) obtained from same incision (List separately in addition to code for primary procedure) Notes: (Use 20936 in conjunction with 22319, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812) A provider uses an autograft, a type of donor bone, to fill in bony defects as she performs a spinal surgery procedure. She extracts the autograft from the patient’s own bone, taken from the same surgical incision. Coding Tips: Code 20936 is an add on code describing grafting from a donor area using the same incision during a major operative procedure and must be reported with an allowable primary spinal procedure code. You should never append modifier 50, Bilateral procedure, to 20936. The CMS Physician Fee Schedule Database includes a 9 indictor in the BILAT SURG column for this code. According to further CMS instructions, a 9 indicator in this column means that the concept of a bilateral surgery with spinal grafting does not apply. +20937 Autograft for spine surgery only (includes harvesting the graft); morselized (through separate skin or fascial incision) (List separately in addition to code for primary procedure) Notes: (Use 20937 in conjunction with 22319, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812) The provider uses an autograft, a type of donor bone, to fill in bony defects as she performs a spinal surgery procedure. She extracts the autograft from the patient’s own body during the surgical procedure, through a separate incision. Coding Tips: Code 20937 is an add on code describing preparation and application of a morselized autograft through a separate skin incision and must be reported with an allowable primary spinal procedure code. *** A vertebral segment describes the basic constituent part into which the spine may be divided. It represents a single complete vertebral bone with its associated articular processes and laminae. A vertebral interspace is the non-bony compartment between two adjacent vertebral bodies which contains the intervertebral disc, and includes the nucleus pulposus, annulus fibrosus, and two cartilaginous endplates. Autograft for spine surgery only (includes harvesting the graft); structural, bicortical or tricortical (through separate skin or fascial incision) (List separately in addition to code for primary procedure) Notes: (Use 20938 in conjunction with 22319, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812) (For aspiration of bone marrow for bone grafting, spine surgery only, use 20939) The provider uses an autograft, a type of donor bone, to fill in bony defects as she performs a spinal surgery procedure. She extracts the autograft from the patient's own body during the surgical procedure, through a separate incision. Reporting Cosurgeries Source: CPT® Assistant July 1996 page 7 Coding Tip Reporting Cosurgeries "We receive many questions concerning how to report surgeries performed by more than one physician. To help you understand the proper coding we present the following information." The General Question "I am a general surgeon who sometimes performs surgeries with other surgeons (cosurgeries), such as orthopedic or neurosurgeons. I open the surgical site, the other surgeon does the definitive portion of the procedure, and then I close. What CPT codes should I report for my services? I have heard from some sources that I should bill for a thoracotomy and wound repair. But other sources have told me to report the same CPT codes as the other surgeon. Which is correct? CPT® ASSISTANT'S REPLY: Here's How to Code: "For situations in which one surgeon performs the opening and closing of a surgery and another physician performs the definitive portion of the procedure, both physicians should report the same CPT codes, and appropriately append either modifier -62 or modifier -66." Illustration A patient's surgery includes arthrodesis of two interspaces of the thoracic spine by anterior interbody technique, with anterior instrumentation of three vertebral segments. Physician "A" performs a thoracotomy at the start of the surgical session, and Physician "B" performs the arthrodesis and spinal instrumentation. Upon completion of the arthrodesis and spinal instrumentation, Physician A closes the operative site. Coding the Illustration (The physicians in the illustration would report the codes indicated below.) Physician A 22556-62 Physician B 22556-62 22558-62 22558-62 22845-62 22845-62 When performing these cosurgeries, it is important to communicate with the other surgeon's office to be certain that you submit the claims properly 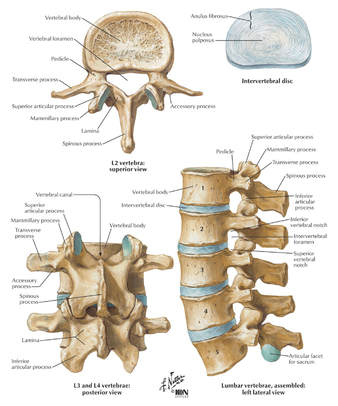 CPT® Guideline September 1997 page 8 Coding Communication How to Code Prosthetic Devices It is not often that we devote an entire article to a single code, but sometimes this is the only way to fully explain the use of certain codes-22851, application of prosthetic device (eg, metal cages, methylmethacrylate) to vertebral defect or interspace, is such a code. But before we review how to report this code, it is probably a good idea to first do a brief anatomical review of the vertebral column. The vertebral column (spine) consists of a series of bones known as vertebrae. An adult human possesses 33 vertebrae divided into the following five types: 7 cervical vertebrae; 12 thoracic vertebrae; 5 lumbar vertebrae; 5 sacral vertebrae; and 4 coccygeal vertebrae. The sacral vertebrae are typically fused into a single bone known as the sacrum. The coccygeal vertebrae are sometimes fused into a single bone known as the coccyx. Therefore, the actual number of bones in the vertebral column may be 26-29, depending on if the coccygeal vertebrae are fused. Vertebrae are commonly named by a letter that corresponds to the region of the vertebral column to which the vertebrae belongs, followed by a number that indicates where in the region the vertebrae is located. For example, the most superior cervical vertebra is called C1, with the next cervical vertebrae down designated C2. The most superior thoracic vertebrae is T1, with the next one down designated T2. Fig. 1 - Spinal Prosthetic Devices Between each pair of vertebrae is a disc that cushions the spinal column. If one of the discs degenerates or if one of the 26-29 vertebrae are injured (as in the case of a fracture, degenerative disease, or secondary to tumor destruction) the physician may need to place a prosthetic device (eg, metal cages or methyl-methacrylate) in the vertebral defect or interspace. (Fig. 1) In these instances, a segment of vertebral level may be drilled and metal cages packed with porous implants of bone graft may be inserted or methylmethacrylate may be placed between the affected vertebrae. Proper Reporting of code 22851 It is important to note that CPT® code 22851 is not intended to be reported per cage. CPT® code 22851 should only be reported one time, regardless if one or more metal cages are placed in the intervertebral space at the same level. However, if metal cages are placed at two different levels, (eg, metal cage placed at L3-4 interspace and L5-S1 interspace), then 22851 may be reported more than once to indicate that one or more cages were placed at two or more different levels. It is important to note that a single cage or methylmethacrylate can cover a defect of several vertebral segments (eg, a single cage may replace three entire vertebrae), wherein code 22851 would still only be reported one time. Within the spine section, instrumentation procedure codes (22840-22855) are reported in addition to the definitive procedure(s) without appending the modifier -51. Therefore, if arthrodesis is performed in addition to the placement of the metal cages, then it would be appropriate to report code 22851 in addition to the appropriate arthrodesis code, 22548-22632. In this instance, the modifier -51 would not be appended to code 22851. If metal cages are placed through an anterior approach and pedicle screws are placed through a posterior approach, it would be appropriate to report both code 22851 and one of the codes from the posterior instrumentation series, 22840, 22842-22844. However, if different instrumentation is used in addition to the metal cages or methylmethacrylate through the same approach (eg, an anterior plating system) or pedicle screws and posterior lumbar interbody fusion utilizing cages), then the appropriate instrumentation code would be reported in addition to code 22851. However, 22851 and 22845 should not both be reported if only the metal cage is inserted. If fracture treatment, dislocation, or arthrodesis is performed in addition to spinal instrumentation, then the appropriate fracture treatment, dislocation or arthrodesis code (22325, 22326, 22327, 22548-22812) would be reported separately in addition to code 22851. In this instance, CPT® code 22851 would be reported in addition to the definitive procedure(s) without the modifier -51 appended. If bone grafting is performed in addition to code 22851, then the appropriate bone grafting code, 20930-20938, would be reported additionally. Clinical Sample: CPT® Code 22851 A 50-year-old man undergoes an anterior fusion of L5-S1 for degenerative disease. A retroperitoneal incision is made and an arthrodesis performed using a BAK cage. A distracter is placed in the interspace, a hole is drilled in the interspace, and the BAK cage is placed in the hole. The spacer is removed and replaced with another BAK cage. Both cages are filled with bone graft. (Report arthrodesis and/or bone grafting separately using the appropriate CPT code[s]). The exposed disk space and adjacent vertebrae are prepared with bone-cutting instruments for acceptance of the prosthetic device. Preparation of the recipient site is made according to the protocol of the particular device. If methylmethacrylate is to be used, a screw or pin may be inserted into the adjacent vertebral surfaces to anchor the methylmethacrylate. Provision is made for cooling of adjacent tissues and protection of heat sensitive tissue from the exothermic reaction of the curing of the methylmethacrylate. For cages, the recipient site is prepared by bone dissection, a trial fit with the device or a spacer or template as indicated by the protocol is inserted and removed for any final modifications of the recipient site. The prosthetic device is then screwed, impacted, or injected into place according to protocol for this particular device. (Additional fixation, other provision for arthrodesis, or bone grafting are coordinated with the placement of the prosthetic device and are coded separately.) For devices that incorporate graft material, that material is appropriately placed into the device prior to its final insertion. CPT® ASSISTANT September 2000 page 10 Coding Consultation Musculoskeletal System, Surgery, 22548-22585, 22899 (Q&A) Question "Should I use the anterior or anterolateral approach technique arthrodesis series of codes (22548-22585) to report intra-abdominal laparoscopic, video assisted anterior interbody fusion?" AMA CPT® Comment "The anterior or anterolateral approach technique arthrodesis series of codes (22548-22585) are intended to describe arthrodesis performed via an open surgical approach. There is not a specific CPT code that accurately describes laparoscopic anterior interbody fusion. Therefore, code 22899, Unlisted procedure, spine should be reported. When reporting an unlisted code to describe a procedure or service, it will be necessary to submit supporting documentation (eg, procedure report) along with the claim to provide an adequate description of the nature, extent, need for the procedure, and the time limit, effort, and equipment necessary to provide the service." CPT® ASSISTANT March 2015 page 9 Frequently Asked Questions:Surgery: Musculoskeletal System Question: "Are CPT codes 22851 and 22845 appropriate to report when modular implants, such as the RSB (RSB LLC; Cleveland, OH) InterPlate® (a modular interbody platform technology), are implanted for spinal fusion procedures?" Answer: "No. The RSB InterPlate® describes a stand-alone interbody fusion device that consists of an interbody spacer with screw fixation or other mechanisms, which engage adjacent vertebrae. Such devices should be reported with code 22558, Arthrodesis, anterior interbody technique, including minimal discectomy to prepare interspace (other than for decompression); lumbar, and 22851, Application of intervertebral biomechanical device(s) (eg, synthetic cage(s), methylmethacrylate) to vertebral defect or interspace (List separately in addition to code for primary procedure). An additional anterior instrumentation code (22845) is not applicable because there is no separate construct placed across the vertebral segment." Question: "Would it be appropriate to separately report any of the following with the hammertoe correction code 28285 (2nd digit), if adequately documented? (1) Resection of hypertrophied base of proximal phalanx (28126), if performed through a separate incision at the metatarsophalangeal (MTP joint); (2) flexor tenotomy (28232) performed through a separate incision at the distal interphalangeal (DIP) joint; (3) an additional unit of 28285 if K-wire is inserted through the DIP, MTP, or proximal interphalangeal (PIP) joint." Answer: "No. Code 28126, Resection, partial or complete, phalangeal base, each toe; code 28232, Tenotomy, open, tendon flexor; toe, single tendon (separate procedure); and the insertion of K-wire through DIP, PIP, and MTP joints are all inclusive components of the procedure described by code 28285, Correction, hammertoe (eg, interphalangeal fusion, partial or total phalangectomy), and should not be reported separately." References: 2020 AMA's CPT® Guidelines 2019 AMA's CPT® Guidelines 2018 AMA's CPT® Guidelines 2017 AMA's CPT® Guidelines CPT® Assistant Archives Websites: NASS Spine-Health Medtronic Ahima AAPC CMS All other commercial payers clinical guidelines from the public domains on the internet Read more blog posts:2017 MODERATE SEDATION CHANGE CODES LISTThe 2017 code set revises this code by removing moderate sedation, also called conscious sedation, from this procedure. Use of moderate (conscious) sedation is no longer considered an inherent (bundled) / part of the procedure and can now be reported separately. Prior to the 2017 change, reimbursement for moderate (conscious) sedation was built into the compensation for the procedure as the anesthesia was administered by the same physician or other qualified health care professional who performed the procedure. This code included conscious sedation as an inherent part of providing the service and was not separately reportable. It has been recognized that practice patterns for some procedures have changed, with anesthesia increasingly reported separately by a provider separate from the one who performs the procedure. For this reason, CPT® 2017 unbundles moderate (conscious) sedation from hundreds of codes. To report moderate (conscious) sedation when provided by the same physician or other qualified health care professional who performs the procedure, see new CPT® 2017 codes 99151, 99152, or 99153. To report moderate (conscious) sedation services provided by a physician or other qualified health care professional other than the provider performing the procedure, see new CPT® 2017 codes 99155, 99156, or 99157. For 2017, existing CPT® codes for moderate sedation, 99143-99150, have been deleted. Here are your Code Descriptions for Moderate Sedation 2017 CPT Changes0200T Percutaneous sacral augmentation (sacroplasty), unilateral injection(s), including the use of a balloon or mechanical device, when used, 1 or more needles, includes imaging guidance and bone biopsy, when performed
0201T Percutaneous sacral augmentation (sacroplasty), bilateral injections, including the use of a balloon or mechanical device, when used, 2 or more needles, includes imaging guidance and bone biopsy, when performed 0293T Insertion of left atrial hemodynamic monitor; complete system, includes implanted communication module and pressure sensor lead in left atrium including transseptal access, radiological supervision and interpretation, and associated injection procedures, when performed 0294T Insertion of left atrial hemodynamic monitor; pressure sensor lead at time of insertion of pacing cardioverter-defibrillator pulse generator including radiological supervision and interpretation and associated injection procedures, when performed (List separately in addition to code for primary procedure) 0301T Destruction/reduction of malignant breast tumor with externally applied focused microwave, including interstitial placement of disposable catheter with combined temperature monitoring probe and microwave focusing sensocatheter under ultrasound thermotherapy guidance 0302T Insertion or removal and replacement of intracardiac ischemia monitoring system including imaging supervision and interpretation when performed and intra-operative interrogation and programming when performed; complete system (includes device and electrode) 0303T Insertion or removal and replacement of intracardiac ischemia monitoring system including imaging supervision and interpretation when performed and intra-operative interrogation and programming when performed; electrode only 0304T Insertion or removal and replacement of intracardiac ischemia monitoring system including imaging supervision and interpretation when performed and intra-operative interrogation and programming when performed; device only 0307T Removal of intracardiac ischemia monitoring device 0308T Insertion of ocular telescope prosthesis including removal of crystalline lens or intraocular lens prosthesis 0335T Extra-osseous subtalar joint implant for talotarsal stabilization 0340T Ablation, pulmonary tumor(s), including pleura or chest wall when involved by tumor extension, percutaneous, cryoablation, unilateral, includes imaging guidance 0397T Endoscopic retrograde cholangiopancreatography (ERCP), with optical endomicroscopy (List separately in addition to code for primary procedure) 10030 Image-guided fluid collection drainage by catheter (eg, abscess, hematoma, seroma, lymphocele, cyst), soft tissue (eg, extremity, abdominal wall, neck), percutaneous 20982 Ablation therapy for reduction or eradication of 1 or more bone tumors (eg, metastasis) including adjacent soft tissue when involved by tumor extension, percutaneous, including imaging guidance when performed; radiofrequency 20983 Ablation therapy for reduction or eradication of 1 or more bone tumors (eg, metastasis) including adjacent soft tissue when involved by tumor extension, percutaneous, including imaging guidance when performed; cryoablation 22510 Percutaneous vertebroplasty (bone biopsy included when performed), 1 vertebral body, unilateral or bilateral injection, inclusive of all imaging guidance; cervicothoracic 22511 Percutaneous vertebroplasty (bone biopsy included when performed), 1 vertebral body, unilateral or bilateral injection, inclusive of all imaging guidance; lumbosacral 22512 Percutaneous vertebroplasty (bone biopsy included when performed), 1 vertebral body, unilateral or bilateral injection, inclusive of all imaging guidance; each additional cervicothoracic or lumbosacral vertebral body (List separately in addition to code for primary procedure) 22513 Percutaneous vertebral augmentation, including cavity creation (fracture reduction and bone biopsy included when performed) using mechanical device (eg, kyphoplasty), 1 vertebral body, unilateral or bilateral cannulation, inclusive of all imaging guidance; thoracic 22514 Percutaneous vertebral augmentation, including cavity creation (fracture reduction and bone biopsy included when performed) using mechanical device (eg, kyphoplasty), 1 vertebral body, unilateral or bilateral cannulation, inclusive of all imaging guidance; lumbar 22515 Percutaneous vertebral augmentation, including cavity creation (fracture reduction and bone biopsy included when performed) using mechanical device (eg, kyphoplasty), 1 vertebral body, unilateral or bilateral cannulation, inclusive of all imaging guidance; each additional thoracic or lumbar vertebral body (List separately in addition to code for primary procedure) 22526 Percutaneous intradiscal electrothermal annuloplasty, unilateral or bilateral including fluoroscopic guidance; single level 22527 Percutaneous intradiscal electrothermal annuloplasty, unilateral or bilateral including fluoroscopic guidance; 1 or more additional levels (List separately in addition to code for primary procedure) 31615 Tracheobronchoscopy through established tracheostomy incision 31622 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; diagnostic, with cell washing, when performed (separate procedure) 31623 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with brushing or protected brushings 31624 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with bronchial alveolar lavage 31625 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with bronchial or endobronchial biopsy(s), single or multiple sites 31626 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with placement of fiducial markers, single or multiple 2017 Moderate Conscious Sedation Changes 31627 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with computer-assisted, image-guided navigation (List separately in addition to code for primary procedure[s]) 31628 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with transbronchial lung biopsy(s), single lobe 31629 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with transbronchial needle aspiration biopsy(s), trachea, main stem and/or lobar bronchus(i) 31632 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with transbronchial lung biopsy(s), each additional lobe (List separately in addition to code for primary procedure) 31633 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with transbronchial needle aspiration biopsy(s), each additional lobe (List separately in addition to code for primary procedure) 31634 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with balloon occlusion, with assessment of air leak, with administration of occlusive substance (eg, fibrin glue), if performed 31635 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with removal of foreign body 31645 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with therapeutic aspiration of tracheobronchial tree, initial (eg, drainage of lung abscess) 31646 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with therapeutic aspiration of tracheobronchial tree, subsequent 31647 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with balloon occlusion, when performed, assessment of air leak, airway sizing, and insertion of bronchial valve(s), initial lobe 31648 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with removal of bronchial valve(s), initial lobe 31649 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with removal of bronchial valve(s), each additional lobe (List separately in addition to code for primary procedure) 31651 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with balloon occlusion, when performed, assessment of air leak, airway sizing, and insertion of bronchial valve(s), each additional lobe (List separately in addition to code for primary procedure[s]) 31652 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with endobronchial ultrasound (EBUS) guided transtracheal and/or transbronchial sampling (eg, aspiration[s]/biopsy[ies]), one or two mediastinal and/or hilar lymph node stations or structures 31653 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with endobronchial ultrasound (EBUS) guided transtracheal and/or transbronchial sampling (eg, aspiration[s]/biopsy[ies]), 3 or more mediastinal and/or hilar lymph node stations or structures 31654 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with transendoscopic endobronchial ultrasound (EBUS) during bronchoscopic diagnostic or therapeutic intervention(s) for peripheral lesion(s) (List separately in addition to code for primary procedure[s]) 31660 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with bronchial thermoplasty, 1 lobe 31661 Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with bronchial thermoplasty, 2 or more lobes 31725 Catheter aspiration (separate procedure); tracheobronchial with fiberscope, bedside 32405 Biopsy, lung or mediastinum, percutaneous needle 32550 Insertion of indwelling tunneled pleural catheter with cuff 32551 Tube thoracostomy, includes connection to drainage system (eg, water seal), when performed, open (separate procedure) 32553 Placement of interstitial device(s) for radiation therapy guidance (eg, fiducial markers, dosimeter), percutaneous, intra-thoracic, single or multiple 33010 Pericardiocentesis; initial 33011 Pericardiocentesis; subsequent 33206 Insertion of new or replacement of permanent pacemaker with transvenous electrode(s); atrial 33207 Insertion of new or replacement of permanent pacemaker with transvenous electrode(s); ventricular 33208 Insertion of new or replacement of permanent pacemaker with transvenous electrode(s); atrial and ventricular 33210 Insertion or replacement of temporary transvenous single chamber cardiac electrode or pacemaker catheter (separate procedure) 33211 Insertion or replacement of temporary transvenous dual chamber pacing electrodes (separate procedure) 33212 Insertion of pacemaker pulse generator only; with existing single lead 33213 Insertion of pacemaker pulse generator only; with existing dual leads 33214 Upgrade of implanted pacemaker system, conversion of single chamber system to dual chamber system (includes removal of previously placed pulse generator, testing of existing lead, insertion of new lead, insertion of new pulse generator) 33216 Insertion of a single transvenous electrode, permanent pacemaker or implantable defibrillator 33217 Insertion of 2 transvenous electrodes, permanent pacemaker or implantable defibrillator 33218 Repair of single transvenous electrode, permanent pacemaker or implantable defibrillator 33220 Repair of 2 transvenous electrodes for permanent pacemaker or implantable defibrillator 33221 Insertion of pacemaker pulse generator only; with existing multiple leads 33222 Relocation of skin pocket for pacemaker 33223 Relocation of skin pocket for implantable defibrillator 33227 Removal of permanent pacemaker pulse generator with replacement of pacemaker pulse generator; single lead system 33228 Removal of permanent pacemaker pulse generator with replacement of pacemaker pulse generator; dual lead system 33229 Removal of permanent pacemaker pulse generator with replacement of pacemaker pulse generator; multiple lead system 33230 Insertion of implantable defibrillator pulse generator only; with existing dual leads 33231 Insertion of implantable defibrillator pulse generator only; with existing multiple leads 33233 Removal of permanent pacemaker pulse generator only 33234 Removal of transvenous pacemaker electrode(s); single lead system, atrial or ventricular 33235 Removal of transvenous pacemaker electrode(s); dual lead system 33240 Insertion of implantable defibrillator pulse generator only; with existing single lead 2017 Moderate Conscious Sedation Changes 33241 Removal of implantable defibrillator pulse generator only 33244 Removal of single or dual chamber implantable defibrillator electrode(s); by transvenous extraction 33249 Insertion or replacement of permanent implantable defibrillator system, with transvenous lead(s), single or dual chamber 33262 Removal of implantable defibrillator pulse generator with replacement of implantable defibrillator pulse generator; single lead system 33263 Removal of implantable defibrillator pulse generator with replacement of implantable defibrillator pulse generator; dual lead system 33264 Removal of implantable defibrillator pulse generator with replacement of implantable defibrillator pulse generator; multiple lead system 33282 Implantation of patient-activated cardiac event recorder 33284 Removal of an implantable, patient-activated cardiac event recorder 33990 Insertion of ventricular assist device, percutaneous including radiological supervision and interpretation; arterial access only 33991 Insertion of ventricular assist device, percutaneous including radiological supervision and interpretation; both arterial and venous access, with transseptal puncture 33992 Removal of percutaneous ventricular assist device at separate and distinct session from insertion 33993 Repositioning of percutaneous ventricular assist device with imaging guidance at separate and distinct session from insertion 36010 Introduction of catheter, superior or inferior vena cava 36140 Introduction of needle or intracatheter; extremity artery 36200 Introduction of catheter, aorta 36221 Non-selective catheter placement, thoracic aorta, with angiography of the extracranial carotid, vertebral, and/or intracranial vessels, unilateral or bilateral, and all associated radiological supervision and interpretation, includes angiography of the cervicocerebral arch, when performed 36222 Selective catheter placement, common carotid or innominate artery, unilateral, any approach, with angiography of the ipsilateral extracranial carotid circulation and all associated radiological supervision and interpretation, includes angiography of the cervicocerebral arch, when performed 36223 Selective catheter placement, common carotid or innominate artery, unilateral, any approach, with angiography of the ipsilateral intracranial carotid circulation and all associated radiological supervision and interpretation, includes angiography of the extracranial carotid and cervicocerebral arch, when performed 36224 Selective catheter placement, internal carotid artery, unilateral, with angiography of the ipsilateral intracranial carotid circulation and all associated radiological supervision and interpretation, includes angiography of the extracranial carotid and cervicocerebral arch, when performed 2017 Moderate Conscious Sedation Changes 36225 Selective catheter placement, subclavian or innominate artery, unilateral, with angiography of the ipsilateral vertebral circulation and all associated radiological supervision and interpretation, includes angiography of the cervicocerebral arch, when performed 36226 Selective catheter placement, vertebral artery, unilateral, with angiography of the ipsilateral vertebral circulation and all associated radiological supervision and interpretation, includes angiography of the cervicocerebral arch, when performed 36227 Selective catheter placement, external carotid artery, unilateral, with angiography of the ipsilateral external carotid circulation and all associated radiological supervision and interpretation (List separately in addition to code for primary procedure) 36228 Selective catheter placement, each intracranial branch of the internal carotid or vertebral arteries, unilateral, with angiography of the selected vessel circulation and all associated radiological supervision and interpretation (eg, middle cerebral artery, posterior inferior cerebellar artery) (List separately in addition to code for primary procedure) 36245 Selective catheter placement, arterial system; each first order abdominal, pelvic, or lower extremity artery branch, within a vascular family 36246 Selective catheter placement, arterial system; initial second order abdominal, pelvic, or lower extremity artery branch, within a vascular family 36247 Selective catheter placement, arterial system; initial third order or more selective abdominal, pelvic, or lower extremity artery branch, within a vascular family 36248 Selective catheter placement, arterial system; additional second order, third order, and beyond, abdominal, pelvic, or lower extremity artery branch, within a vascular family (List in addition to code for initial second or third order vessel as appropriate) 36251 Selective catheter placement (first-order), main renal artery and any accessory renal artery(s) for renal angiography, including arterial puncture and catheter placement(s), fluoroscopy, contrast injection(s), image postprocessing, permanent recording of images, and radiological supervision and interpretation, including pressure gradient measurements when performed, and flush aortogram when performed; unilateral 36252 Selective catheter placement (first-order), main renal artery and any accessory renal artery(s) for renal angiography, including arterial puncture and catheter placement(s), fluoroscopy, contrast injection(s), image postprocessing, permanent recording of images, and radiological supervision and interpretation, including pressure gradient measurements when performed, and flush aortogram when performed; bilateral 36253 Superselective catheter placement (one or more second order or higher renal artery branches) renal artery and any accessory renal artery(s) for renal angiography, including arterial puncture, catheterization, fluoroscopy, contrast injection(s), image postprocessing, permanent recording of images, and radiological supervision and interpretation, including pressure gradient measurements when performed, and flush aortogram when performed; unilateral 36254 Superselective catheter placement (one or more second order or higher renal artery branches) renal artery and any accessory renal artery(s) for renal angiography, including arterial puncture, catheterization, fluoroscopy, contrast injection(s), image postprocessing, permanent recording of images, and radiological supervision and interpretation, including pressure gradient measurements when performed, and flush aortogram when performed; bilateral 36481 Percutaneous portal vein catheterization by any method 36555 Insertion of non-tunneled centrally inserted central venous catheter; younger than 5 years of age 36557 Insertion of tunneled centrally inserted central venous catheter, without subcutaneous port or pump; younger than 5 years of age 36558 Insertion of tunneled centrally inserted central venous catheter, without subcutaneous port or pump; age 5 years or older 36560 Insertion of tunneled centrally inserted central venous access device, with subcutaneous port; younger than 5 years of age 36561 Insertion of tunneled centrally inserted central venous access device, with subcutaneous port; age 5 years or older 36563 Insertion of tunneled centrally inserted central venous access device with subcutaneous pump 36565 Insertion of tunneled centrally inserted central venous access device, requiring 2 catheters via 2 separate venous access sites; without subcutaneous port or pump (eg, Tesio type catheter) 36566 Insertion of tunneled centrally inserted central venous access device, requiring 2 catheters via 2 separate venous access sites; with subcutaneous port(s) 36568 Insertion of peripherally inserted central venous catheter (PICC), without subcutaneous port or pump; younger than 5 years of age 2017 CPT Code Updates (New, Revised and Deleted) – Moderate Conscious Sedation Changes 36570 Insertion of peripherally inserted central venous access device, with subcutaneous port; younger than 5 years of age 36571 Insertion of peripherally inserted central venous access device, with subcutaneous port; age 5 years or older 36576 Repair of central venous access device, with subcutaneous port or pump, central or peripheral insertion site 36578 Replacement, catheter only, of central venous access device, with subcutaneous port or pump, central or peripheral insertion site 36581 Replacement, complete, of a tunneled centrally inserted central venous catheter, without subcutaneous port or pump, through same venous access 36582 Replacement, complete, of a tunneled centrally inserted central venous access device, with subcutaneous port, through same venous access 36583 Replacement, complete, of a tunneled centrally inserted central venous access device, with subcutaneous pump, through same venous access 36585 Replacement, complete, of a peripherally inserted central venous access device, with subcutaneous port, through same venous access 36590 Removal of tunneled central venous access device, with subcutaneous port or pump, central or peripheral insertion 37183 Revision of transvenous intrahepatic portosystemic shunt(s) (TIPS) (includes venous access, hepatic and portal vein catheterization, portography with hemodynamic evaluation, intrahepatic tract recanulization/dilatation, stent placement and all associated imaging guidance and documentation) 37184 Primary percutaneous transluminal mechanical thrombectomy, noncoronary, non-intracranial, arterial or arterial bypass graft, including fluoroscopic guidance and intraprocedural pharmacological thrombolytic injection(s); initial vessel 37185 Primary percutaneous transluminal mechanical thrombectomy, noncoronary, non-intracranial, arterial or arterial bypass graft, including fluoroscopic guidance and intraprocedural pharmacological thrombolytic injection(s); second and all subsequent vessel(s) within the same vascular family (List separately in addition to code for primary mechanical thrombectomy procedure) 37186 Secondary percutaneous transluminal thrombectomy (eg, nonprimary mechanical, snare basket, suction technique), noncoronary, non-intracranial, arterial or arterial bypass graft, including fluoroscopic guidance and intraprocedural pharmacological thrombolytic injections, provided in conjunction with another percutaneous intervention other than primary mechanical thrombectomy (List separately in addition to code for primary procedure) 37187 Percutaneous transluminal mechanical thrombectomy, vein(s), including intraprocedural pharmacological thrombolytic injections and fluoroscopic guidance 37188 Percutaneous transluminal mechanical thrombectomy, vein(s), including intraprocedural pharmacological thrombolytic injections and fluoroscopic guidance, repeat treatment on subsequent day during course of thrombolytic therapy 37191 Insertion of intravascular vena cava filter, endovascular approach including vascular access, vessel selection, and radiological supervision and interpretation, intraprocedural roadmapping, and imaging guidance (ultrasound and fluoroscopy), when performed 37192 Repositioning of intravascular vena cava filter, endovascular approach including vascular access, vessel selection, and radiological supervision and interpretation, intraprocedural roadmapping, and imaging guidance (ultrasound and fluoroscopy), when performed 37193 Retrieval (removal) of intravascular vena cava filter, endovascular approach including vascular access, vessel selection, and radiological supervision and interpretation, intraprocedural roadmapping, and imaging guidance (ultrasound and fluoroscopy), when performed 37197 Transcatheter retrieval, percutaneous, of intravascular foreign body (eg, fractured venous or arterial catheter), includes radiological supervision and interpretation, and imaging guidance (ultrasound or fluoroscopy), when performed 37211 Transcatheter therapy, arterial infusion for thrombolysis other than coronary or intracranial, any method, including radiological supervision and interpretation, initial treatment day 37212 Transcatheter therapy, venous infusion for thrombolysis, any method, including radiological supervision and interpretation, initial treatment day 37213 Transcatheter therapy, arterial or venous infusion for thrombolysis other than coronary, any method, including radiological supervision and interpretation, continued treatment on subsequent day during course of thrombolytic therapy, including follow-up catheter contrast injection, position change, or exchange, when performed 37214 Transcatheter therapy, arterial or venous infusion for thrombolysis other than coronary, any method, including radiological supervision and interpretation, continued treatment on subsequent day during course of thrombolytic therapy, including follow-up catheter contrast injection, position change, or exchange, when performed; cessation of thrombolysis including removal of catheter and vessel closure by any method 37215 Transcatheter placement of intravascular stent(s), cervical carotid artery, open or percutaneous, including angioplasty, when performed, and radiological supervision and interpretation; with distal embolic protection 37216 Transcatheter placement of intravascular stent(s), cervical carotid artery, open or percutaneous, including angioplasty, when performed, and radiological supervision and interpretation; without distal embolic protection 37218 Transcatheter placement of intravascular stent(s), intrathoracic common carotid artery or innominate artery, open or percutaneous antegrade approach, including angioplasty, when performed, and radiological supervision and interpretation 37220 Revascularization, endovascular, open or percutaneous, iliac artery, unilateral, initial vessel; with transluminal angioplasty 37221 Revascularization, endovascular, open or percutaneous, iliac artery, unilateral, initial vessel; with transluminal stent placement(s), includes angioplasty within the same vessel, when performed 37222 Revascularization, endovascular, open or percutaneous, iliac artery, each additional ipsilateral iliac vessel; with transluminal angioplasty (List separately in addition to code for primary procedure) 37223 Revascularization, endovascular, open or percutaneous, iliac artery, each additional ipsilateral iliac vessel; with transluminal stent placement(s), includes angioplasty within the same vessel, when performed (List separately in addition to code for primary procedure) 37224 Revascularization, endovascular, open or percutaneous, femoral, popliteal artery(s), unilateral; with transluminal angioplasty 37225 Revascularization, endovascular, open or percutaneous, femoral, popliteal artery(s), unilateral; with atherectomy, includes angioplasty within the same vessel, when performed 37226 Revascularization, endovascular, open or percutaneous, femoral, popliteal artery(s), unilateral; with transluminal stent placement(s), includes angioplasty within the same vessel, when performed 37227 Revascularization, endovascular, open or percutaneous, femoral, popliteal artery(s), unilateral; with transluminal stent placement(s) and atherectomy, includes angioplasty within the same vessel, when performed 37228 Revascularization, endovascular, open or percutaneous, tibial, peroneal artery, unilateral, initial vessel; with transluminal angioplasty 37229 Revascularization, endovascular, open or percutaneous, tibial, peroneal artery, unilateral, initial vessel; with atherectomy, includes angioplasty within the same vessel, when performed 37230 Revascularization, endovascular, open or percutaneous, tibial, peroneal artery, unilateral, initial vessel; with transluminal stent placement(s), includes angioplasty within the same vessel, when performed 37231 Revascularization, endovascular, open or percutaneous, tibial, peroneal artery, unilateral, initial vessel; with transluminal stent placement(s) and atherectomy, includes angioplasty within the same vessel, when performed 2017 Moderate Conscious Sedation Changes (continue reading below) 37232 Revascularization, endovascular, open or percutaneous, tibial/peroneal artery, unilateral, each additional vessel; with transluminal angioplasty (List separately in addition to code for primary procedure) 37233 Revascularization, endovascular, open or percutaneous, tibial/peroneal artery, unilateral, each additional vessel; with atherectomy, includes angioplasty within the same vessel, when performed (List separately in addition to code for primary procedure) 37234 Revascularization, endovascular, open or percutaneous, tibial/peroneal artery, unilateral, each additional vessel; with transluminal stent placement(s), includes angioplasty within the same vessel, when performed (List separately in addition to code for primary procedure) 37235 Revascularization, endovascular, open or percutaneous, tibial/peroneal artery, unilateral, each additional vessel; with transluminal stent placement(s) and atherectomy, includes angioplasty within the same vessel, when performed (List separately in addition to code for primary procedure) 37236 Transcatheter placement of an intravascular stent(s) (except lower extremity artery(s) for occlusive disease, cervical carotid, extracranial vertebral or intrathoracic carotid, intracranial, or coronary), open or percutaneous, including radiological supervision and interpretation and including all angioplasty within the same vessel, when performed; initial artery 37237 Transcatheter placement of an intravascular stent(s) (except lower extremity artery(s) for occlusive disease, cervical carotid, extracranial vertebral or intrathoracic carotid, intracranial, or coronary), open or percutaneous, including radiological supervision and interpretation and including all angioplasty within the same vessel, when performed; each additional artery (List separately in addition to code for primary procedure) 37238 Transcatheter placement of an intravascular stent(s), open or percutaneous, including radiological supervision and interpretation and including angioplasty within the same vessel, when performed; initial vein 37239 Transcatheter placement of an intravascular stent(s), open or percutaneous, including radiological supervision and interpretation and including angioplasty within the same vessel, when performed; each additional vein (List separately in addition to code for primary procedure) 37241 Vascular embolization or occlusion, inclusive of all radiological supervision and interpretation, intraprocedural roadmapping, and imaging guidance necessary to complete the intervention; venous, other than hemorrhage (eg, congenital or acquired venous malformations, venous and capillary hemangiomas, varices, varicoceles) 37242 Vascular embolization or occlusion, inclusive of all radiological supervision and interpretation, intraprocedural roadmapping, and imaging guidance necessary to complete the intervention; arterial, other than hemorrhage or tumor (eg, congenital or acquired arterial malformations, arteriovenous malformations, arteriovenous fistulas, aneurysms, pseudoaneurysms) 37243 Vascular embolization or occlusion, inclusive of all radiological supervision and interpretation, intraprocedural roadmapping, and imaging guidance necessary to complete the intervention; for tumors, organ ischemia, or infarction 37244 Vascular embolization or occlusion, inclusive of all radiological supervision and interpretation, intraprocedural roadmapping, and imaging guidance necessary to complete the intervention; for arterial or venous hemorrhage or lymphatic extravasation 37252 Intravascular ultrasound (noncoronary vessel) during diagnostic evaluation and/or therapeutic intervention, including radiological supervision and interpretation; initial noncoronary vessel (List separately in addition to code for primary procedure) 37253 Intravascular ultrasound (noncoronary vessel) during diagnostic evaluation and/or therapeutic intervention, including radiological supervision and interpretation; each additional noncoronary vessel (List separately in addition to code for primary procedure) 43200 Esophagoscopy, flexible, transoral; diagnostic, including collection of specimen(s) by brushing or washing, when performed (separate procedure) 43201 Esophagoscopy, flexible, transoral; with directed submucosal injection(s), any substance 43202 Esophagoscopy, flexible, transoral; with biopsy, single or multiple 43204 Esophagoscopy, flexible, transoral; with injection sclerosis of esophageal varices 43205 Esophagoscopy, flexible, transoral; with band ligation of esophageal varices 43206 Esophagoscopy, flexible, transoral; with optical endomicroscopy 43211 Esophagoscopy, flexible, transoral; with endoscopic mucosal resection 43212 Esophagoscopy, flexible, transoral; with placement of endoscopic stent (includes pre- and post-dilation and guide wire passage, when performed) 43213 Esophagoscopy, flexible, transoral; with dilation of esophagus, by balloon or dilator, retrograde (includes fluoroscopic guidance, when performed) 43214 Esophagoscopy, flexible, transoral; with dilation of esophagus with balloon (30 mm diameter or larger) (includes fluoroscopic guidance, when performed) 43215 Esophagoscopy, flexible, transoral; with removal of foreign body(s) 43216 Esophagoscopy, flexible, transoral; with removal of tumor(s), polyp(s), or other lesion(s) by hot biopsy forceps 43217 Esophagoscopy, flexible, transoral; with removal of tumor(s), polyp(s), or other lesion(s) by snare technique 43220 Esophagoscopy, flexible, transoral; with transendoscopic balloon dilation (less than 30 mm diameter) 43226 Esophagoscopy, flexible, transoral; with insertion of guide wire followed by passage of dilator(s) over guide wire 43227 Esophagoscopy, flexible, transoral; with control of bleeding, any method 43229 Esophagoscopy, flexible, transoral; with ablation of tumor(s), polyp(s), or other lesion(s) (includes pre- and post-dilation and guide wire passage, when performed) 43231 Esophagoscopy, flexible, transoral; with endoscopic ultrasound examination 43232 Esophagoscopy, flexible, transoral; with transendoscopic ultrasound-guided intramural or transmural fine needle aspiration/biopsy(s) 43233 Esophagogastroduodenoscopy, flexible, transoral; with dilation of esophagus with balloon (30 mm diameter or larger) (includes fluoroscopic guidance, when performed) 43235 Esophagogastroduodenoscopy, flexible, transoral; diagnostic, including collection of specimen(s) by brushing or washing, when performed (separate procedure) 43236 Esophagogastroduodenoscopy, flexible, transoral; with directed submucosal injection(s), any substance 43237 Esophagogastroduodenoscopy, flexible, transoral; with endoscopic ultrasound examination limited to the esophagus, stomach or duodenum, and adjacent structures 43238 Esophagogastroduodenoscopy, flexible, transoral; with transendoscopic ultrasound-guided intramural or transmural fine needle aspiration/biopsy(s), (includes endoscopic ultrasound examination limited to the esophagus, stomach or duodenum, and adjacent structures) 43239 Esophagogastroduodenoscopy, flexible, transoral; with biopsy, single or multiple 43240 Esophagogastroduodenoscopy, flexible, transoral; with transmural drainage of pseudocyst (includes placement of transmural drainage catheter[s]/stent[s], when performed, and endoscopic ultrasound, when performed) 43241 Esophagogastroduodenoscopy, flexible, transoral; with insertion of intraluminal tube or catheter 43242 Esophagogastroduodenoscopy, flexible, transoral; with transendoscopic ultrasound-guided intramural or transmural fine needle aspiration/biopsy(s) (includes endoscopic ultrasound examination of the esophagus, stomach, and either the duodenum or a surgically altered stomach where the jejunum is examined distal to the anastomosis) 43243 Esophagogastroduodenoscopy, flexible, transoral; with injection sclerosis of esophageal/gastric varices 43244 Esophagogastroduodenoscopy, flexible, transoral; with band ligation of esophageal/gastric varices 43245 Esophagogastroduodenoscopy, flexible, transoral; with dilation of gastric/duodenal stricture(s) (eg, balloon, bougie) 43246 Esophagogastroduodenoscopy, flexible, transoral; with directed placement of percutaneous gastrostomy tube 43247 Esophagogastroduodenoscopy, flexible, transoral; with removal of foreign body(s) 43248 Esophagogastroduodenoscopy, flexible, transoral; with insertion of guide wire followed by passage of dilator(s) through esophagus over guide wire 43249 Esophagogastroduodenoscopy, flexible, transoral; with transendoscopic balloon dilation of esophagus (less than 30 mm diameter) 43250 Esophagogastroduodenoscopy, flexible, transoral; with removal of tumor(s), polyp(s), or other lesion(s) by hot biopsy forceps 43251 Esophagogastroduodenoscopy, flexible, transoral; with removal of tumor(s), polyp(s), or other lesion(s) by snare technique 43252 Esophagogastroduodenoscopy, flexible, transoral; with optical endomicroscopy 43253 Esophagogastroduodenoscopy, flexible, transoral; with transendoscopic ultrasound-guided transmural injection of diagnostic or therapeutic substance(s) (eg, anesthetic, neurolytic agent) or fiducial marker(s) (includes endoscopic ultrasound examination of the esophagus, stomach, and either the duodenum or a surgically altered stomach where the jejunum is examined distal to the anastomosis) 43254 Esophagogastroduodenoscopy, flexible, transoral; with endoscopic mucosal resection 43255 Esophagogastroduodenoscopy, flexible, transoral; with control of bleeding, any method 43257 Esophagogastroduodenoscopy, flexible, transoral; with delivery of thermal energy to the muscle of lower esophageal sphincter and/or gastric cardia, for treatment of gastroesophageal reflux disease 43259 Esophagogastroduodenoscopy, flexible, transoral; with endoscopic ultrasound examination, including the esophagus, stomach, and either the duodenum or a surgically altered stomach where the jejunum is examined distal to the anastomosis 43260 Endoscopic retrograde cholangiopancreatography (ERCP); diagnostic, including collection of specimen(s) by brushing or washing, when performed (separate procedure) 43261 Endoscopic retrograde cholangiopancreatography (ERCP); with biopsy, single or multiple 43262 Endoscopic retrograde cholangiopancreatography (ERCP); with sphincterotomy/papillotomy 43263 Endoscopic retrograde cholangiopancreatography (ERCP); with pressure measurement of sphincter of Oddi 43264 Endoscopic retrograde cholangiopancreatography (ERCP); with removal of calculi/debris from biliary/pancreatic duct(s) 43265 Endoscopic retrograde cholangiopancreatography (ERCP); with destruction of calculi, any method (eg, mechanical, electrohydraulic, lithotripsy) 43266 Esophagogastroduodenoscopy, flexible, transoral; with placement of endoscopic stent (includes pre- and post-dilation and guide wire passage, when performed) 43270 Esophagogastroduodenoscopy, flexible, transoral; with ablation of tumor(s), polyp(s), or other lesion(s) (includes pre- and post-dilation and guide wire passage, when performed) 43273 Endoscopic cannulation of papilla with direct visualization of pancreatic/common bile duct(s) (List separately in addition to code(s) for primary procedure) 43274 Endoscopic retrograde cholangiopancreatography (ERCP); with placement of endoscopic stent into biliary or pancreatic duct, including pre- and postdilation and guide wire passage, when performed, including sphincterotomy, when performed, each stent 43275 Endoscopic retrograde cholangiopancreatography (ERCP); with removal of foreign body(s) or stent(s) from biliary/pancreatic duct(s) 43276 Endoscopic retrograde cholangiopancreatography (ERCP); with removal and exchange of stent(s), biliary or pancreatic duct, including pre- and postdilation and guide wire passage, when performed, including sphincterotomy, when performed, each stent exchanged 43277 Endoscopic retrograde cholangiopancreatography (ERCP); with trans-endoscopic balloon dilation of biliary/pancreatic duct(s) or of ampulla (sphincteroplasty), including sphincterotomy, when performed, each duct 43278 Endoscopic retrograde cholangiopancreatography (ERCP); with ablation of tumor(s), polyp(s), or other lesion(s), including pre- and post-dilation and guide wire passage, when performed 43453 Dilation of esophagus, over guide wire 44360 Small intestinal endoscopy, enteroscopy beyond second portion of duodenum, not including ileum; diagnostic, including collection of specimen(s) by brushing or washing, when performed (separate procedure) 44361 Small intestinal endoscopy, enteroscopy beyond second portion of duodenum, not including ileum; with biopsy, single or multiple 44363 Small intestinal endoscopy, enteroscopy beyond second portion of duodenum, not including ileum; with removal of foreign body(s) 44364 Small intestinal endoscopy, enteroscopy beyond second portion of duodenum, not including ileum; with removal of tumor(s), polyp(s), or other lesion(s) by snare technique 44365 Small intestinal endoscopy, enteroscopy beyond second portion of duodenum, not including ileum; with removal of tumor(s), polyp(s), or other lesion(s) by hot biopsy forceps or bipolar cautery 44366 Small intestinal endoscopy, enteroscopy beyond second portion of duodenum, not including ileum; with control of bleeding (eg, injection, bipolar cautery, unipolar cautery, laser, heater probe, stapler, plasma coagulator) 44369 Small intestinal endoscopy, enteroscopy beyond second portion of duodenum, not including ileum; with ablation of tumor(s), polyp(s), or other lesion(s) not amenable to removal by hot biopsy forceps, bipolar cautery or snare technique 44370 Small intestinal endoscopy, enteroscopy beyond second portion of duodenum, not including ileum; with transendoscopic stent placement (includes predilation) 44372 Small intestinal endoscopy, enteroscopy beyond second portion of duodenum, not including ileum; with placement of percutaneous jejunostomy tube 44373 Small intestinal endoscopy, enteroscopy beyond second portion of duodenum, not including ileum; with conversion of percutaneous gastrostomy tube to percutaneous jejunostomy tube 44376 Small intestinal endoscopy, enteroscopy beyond second portion of duodenum, including ileum; diagnostic, with or without collection of specimen(s) by brushing or washing (separate procedure) 44377 Small intestinal endoscopy, enteroscopy beyond second portion of duodenum, including ileum; with biopsy, single or multiple 2017 Moderate Conscious Sedation Changes 44378 Small intestinal endoscopy, enteroscopy beyond second portion of duodenum, including ileum; with control of bleeding (eg, injection, bipolar cautery, unipolar cautery, laser, heater probe, stapler, plasma coagulator) 44379 Small intestinal endoscopy, enteroscopy beyond second portion of duodenum, including ileum; with transendoscopic stent placement (includes predilation) 44380 Ileoscopy, through stoma; diagnostic, including collection of specimen(s) by brushing or washing, when performed (separate procedure) 44381 Ileoscopy, through stoma; with transendoscopic balloon dilation 44382 Ileoscopy, through stoma; with biopsy, single or multiple 44384 Ileoscopy, through stoma; with placement of endoscopic stent (includes pre- and post-dilation and guide wire passage, when performed) 44385 Endoscopic evaluation of small intestinal pouch (eg, Kock pouch, ileal reservoir [S or J]); diagnostic, including collection of specimen(s) by brushing or washing, when performed (separate procedure) 44386 Endoscopic evaluation of small intestinal pouch (eg, Kock pouch, ileal reservoir [S or J]); with biopsy, single or multiple 44388 Colonoscopy through stoma; diagnostic, including collection of specimen(s) by brushing or washing, when performed (separate procedure) 44389 Colonoscopy through stoma; with biopsy, single or multiple 44390 Colonoscopy through stoma; with removal of foreign body(s) 44391 Colonoscopy through stoma; with control of bleeding, any method 44392 Colonoscopy through stoma; with removal of tumor(s), polyp(s), or other lesion(s) by hot biopsy forceps 44394 Colonoscopy through stoma; with removal of tumor(s), polyp(s), or other lesion(s) by snare technique 44401 Colonoscopy through stoma; with ablation of tumor(s), polyp(s), or other lesion(s) (includes pre-and post-dilation and guide wire passage, when performed) 44402 Colonoscopy through stoma; with endoscopic stent placement (including pre- and post-dilation and guide wire passage, when performed) 44403 Colonoscopy through stoma; with endoscopic mucosal resection 44404 Colonoscopy through stoma; with directed submucosal injection(s), any substance 44405 Colonoscopy through stoma; with transendoscopic balloon dilation 44406 Colonoscopy through stoma; with endoscopic ultrasound examination, limited to the sigmoid, descending, transverse, or ascending colon and cecum and adjacent structures 44407 Colonoscopy through stoma; with transendoscopic ultrasound guided intramural or transmural fine needle aspiration/biopsy(s), includes endoscopic ultrasound examination limited to the sigmoid, descending, transverse, or ascending colon and cecum and adjacent structures 44408 Colonoscopy through stoma; with decompression (for pathologic distention) (eg, volvulus, megacolon), including placement of decompression tube, when performed 44500 Introduction of long gastrointestinal tube (eg, Miller-Abbott) (separate procedure) 45303 Proctosigmoidoscopy, rigid; with dilation (eg, balloon, guide wire, bougie) 45305 Proctosigmoidoscopy, rigid; with biopsy, single or multiple 45307 Proctosigmoidoscopy, rigid; with removal of foreign body 45308 Proctosigmoidoscopy, rigid; with removal of single tumor, polyp, or other lesion by hot biopsy forceps or bipolar cautery 45309 Proctosigmoidoscopy, rigid; with removal of single tumor, polyp, or other lesion by snare technique 45315 Proctosigmoidoscopy, rigid; with removal of multiple tumors, polyps, or other lesions by hot biopsy forceps, bipolar cautery or snare technique 45317 Proctosigmoidoscopy, rigid; with control of bleeding (eg, injection, bipolar cautery, unipolar cautery, laser, heater probe, stapler, plasma coagulator) 2017 Moderate Conscious Sedation Changes45320 Proctosigmoidoscopy, rigid; with ablation of tumor(s), polyp(s), or other lesion(s) not amenable to removal by hot biopsy forceps, bipolar cautery or snare technique (eg, laser) 45321 Proctosigmoidoscopy, rigid; with decompression of volvulus 45327 Proctosigmoidoscopy, rigid; with transendoscopic stent placement (includes predilation) 45332 Sigmoidoscopy, flexible; with removal of foreign body(s) 45333 Sigmoidoscopy, flexible; with removal of tumor(s), polyp(s), or other lesion(s) by hot biopsy forceps 45334 Sigmoidoscopy, flexible; with control of bleeding, any method 45335 Sigmoidoscopy, flexible; with directed submucosal injection(s), any substance 45337 Sigmoidoscopy, flexible; with decompression (for pathologic distention) (eg, volvulus, megacolon), including placement of decompression tube, when performed 45338 Sigmoidoscopy, flexible; with removal of tumor(s), polyp(s), or other lesion(s) by snare technique 45340 Sigmoidoscopy, flexible; with transendoscopic balloon dilation 45341 Sigmoidoscopy, flexible; with endoscopic ultrasound examination 45342 Sigmoidoscopy, flexible; with transendoscopic ultrasound guided intramural or transmural fine needle aspiration/biopsy(s) 45346 Sigmoidoscopy, flexible; with ablation of tumor(s), polyp(s), or other lesion(s) (includes pre- and post-dilation and guide wire passage, when performed) 45347 Sigmoidoscopy, flexible; with placement of endoscopic stent (includes pre- and post-dilation and guide wire passage, when performed) 45349 Sigmoidoscopy, flexible; with endoscopic mucosal resection 45350 Sigmoidoscopy, flexible; with band ligation(s) (eg, hemorrhoids) 45378 Colonoscopy, flexible; diagnostic, including collection of specimen(s) by brushing or washing, when performed (separate procedure) 45379 Colonoscopy, flexible; with removal of foreign body(s) 45380 Colonoscopy, flexible; with biopsy, single or multiple 45381 Colonoscopy, flexible; with directed submucosal injection(s), any substance 45382 Colonoscopy, flexible; with control of bleeding, any method 45384 Colonoscopy, flexible; with removal of tumor(s), polyp(s), or other lesion(s) by hot biopsy forceps 45385 Colonoscopy, flexible; with removal of tumor(s), polyp(s), or other lesion(s) by snare technique 45386 Colonoscopy, flexible; with transendoscopic balloon dilation 45388 Colonoscopy, flexible; with ablation of tumor(s), polyp(s), or other lesion(s) (includes pre- and post-dilation and guide wire passage, when performed) 45389 Colonoscopy, flexible; with endoscopic stent placement (includes pre- and post-dilation and guide wire passage, when performed) 45390 Colonoscopy, flexible; with endoscopic mucosal resection 45391 Colonoscopy, flexible; with endoscopic ultrasound examination limited to the rectum, sigmoid, descending, transverse, or ascending colon and cecum, and adjacent structures 45392 Colonoscopy, flexible; with transendoscopic ultrasound guided intramural or transmural fine needle aspiration/biopsy(s), includes endoscopic ultrasound examination limited to the rectum, sigmoid, descending, transverse, or ascending colon and cecum, and adjacent structures 45393 Colonoscopy, flexible; with decompression (for pathologic distention) (eg, volvulus, megacolon), including placement of decompression tube, when performed 45398 Colonoscopy, flexible; with band ligation(s) (eg, hemorrhoids) 2017 Moderate Conscious Sedation Changes (continue reading below) 47000 Biopsy of liver, needle; percutaneous 47382 Ablation, 1 or more liver tumor(s), percutaneous, radiofrequency 47383 Ablation, 1 or more liver tumor(s), percutaneous, cryoablation 47532 Injection procedure for cholangiography, percutaneous, complete diagnostic procedure including imaging guidance (eg, ultrasound and/or fluoroscopy) and all associated radiological supervision and interpretation; new access (eg, percutaneous transhepatic cholangiogram) 47533 Placement of biliary drainage catheter, percutaneous, including diagnostic cholangiography when performed, imaging guidance (eg, ultrasound and/or fluoroscopy), and all associated radiological supervision and interpretation; external 47534 Placement of biliary drainage catheter, percutaneous, including diagnostic cholangiography when performed, imaging guidance (eg, ultrasound and/or fluoroscopy), and all associated radiological supervision and interpretation; internal-external 47535 Conversion of external biliary drainage catheter to internal-external biliary drainage catheter, percutaneous, including diagnostic cholangiography when performed, imaging guidance (eg, fluoroscopy), and all associated radiological supervision and interpretation 47536 Exchange of biliary drainage catheter (eg, external, internal-external, or conversion of internal-external to external only), percutaneous, including diagnostic cholangiography when performed, imaging guidance (eg, fluoroscopy), and all associated radiological supervision and interpretation 47541 Placement of access through the biliary tree and into small bowel to assist with an endoscopic biliary procedure (eg, rendezvous procedure), percutaneous, including diagnostic cholangiography when performed, imaging guidance (eg, ultrasound and/or fluoroscopy), and all associated radiological supervision and interpretation, new access 47542 Balloon dilation of biliary duct(s) or of ampulla (sphincteroplasty), percutaneous, including imaging guidance (eg, fluoroscopy), and all associated radiological supervision and interpretation, each duct (List separately in addition to code for primary procedure) 47543 Endoluminal biopsy(ies) of biliary tree, percutaneous, any method(s) (eg, brush, forceps, and/or needle), including imaging guidance (eg, fluoroscopy), and all associated radiological supervision and interpretation, single or multiple (List separately in addition to code for primary procedure) 47544 Removal of calculi/debris from biliary duct(s) and/or gallbladder, percutaneous, including destruction of calculi by any method (eg, mechanical, electrohydraulic, lithotripsy) when performed, imaging guidance (eg, fluoroscopy), and all associated radiological supervision and interpretation (List separately in addition to code for primary procedure) 49405 Image-guided fluid collection drainage by catheter (eg, abscess, hematoma, seroma, lymphocele, cyst); visceral (eg, kidney, liver, spleen, lung/mediastinum), percutaneous 49406 Image-guided fluid collection drainage by catheter (eg, abscess, hematoma, seroma, lymphocele, cyst); peritoneal or retroperitoneal, percutaneous 49407 Image-guided fluid collection drainage by catheter (eg, abscess, hematoma, seroma, lymphocele, cyst); peritoneal or retroperitoneal, transvaginal or transrectal 49411 Placement of interstitial device(s) for radiation therapy guidance (eg, fiducial markers, dosimeter), percutaneous, intra-abdominal, intra-pelvic (except prostate), and/or retroperitoneum, single or multiple 49418 Insertion of tunneled intraperitoneal catheter (eg, dialysis, intraperitoneal chemotherapy instillation, management of ascites), complete procedure, including imaging guidance, catheter placement, contrast injection when performed, and radiological supervision and interpretation, percutaneous 49440 Insertion of gastrostomy tube, percutaneous, under fluoroscopic guidance including contrast injection(s), image documentation and report 49441 Insertion of duodenostomy or jejunostomy tube, percutaneous, under fluoroscopic guidance including contrast injection(s), image documentation and report 49442 Insertion of cecostomy or other colonic tube, percutaneous, under fluoroscopic guidance including contrast injection(s), image documentation and report 49446 Conversion of gastrostomy tube to gastro-jejunostomy tube, percutaneous, under fluoroscopic guidance including contrast injection(s), image documentation and report 50200 Renal biopsy; percutaneous, by trocar or needle 50382 Removal (via snare/capture) and replacement of internally dwelling ureteral stent via percutaneous approach, including radiological supervision and interpretation 50384 Removal (via snare/capture) of internally dwelling ureteral stent via percutaneous approach, including radiological supervision and interpretation 50385 Removal (via snare/capture) and replacement of internally dwelling ureteral stent via transurethral approach, without use of cystoscopy, including radiological supervision and interpretation 50386 Removal (via snare/capture) of internally dwelling ureteral stent via transurethral approach, without use of cystoscopy, including radiological supervision and interpretation 50387 Removal and replacement of externally accessible nephroureteral catheter (eg, external/internal stent) requiring fluoroscopic guidance, including radiological supervision and interpretation 50430 Injection procedure for antegrade nephrostogram and/or ureterogram, complete diagnostic procedure including imaging guidance (eg, ultrasound and fluoroscopy) and all associated radiological supervision and interpretation; new access 50432 Placement of nephrostomy catheter, percutaneous, including diagnostic nephrostogram and/or ureterogram when performed, imaging guidance (eg, ultrasound and/or fluoroscopy) and all associated radiological supervision and interpretation 50433 Placement of nephroureteral catheter, percutaneous, including diagnostic nephrostogram and/or ureterogram when performed, imaging guidance (eg, ultrasound and/or fluoroscopy) and all associated radiological supervision and interpretation, new access 50434 Convert nephrostomy catheter to nephroureteral catheter, percutaneous, including diagnostic nephrostogram and/or ureterogram when performed, imaging guidance (eg, ultrasound and/or fluoroscopy) and all associated radiological supervision and interpretation, via pre-existing nephrostomy tract 50592 Ablation, 1 or more renal tumor(s), percutaneous, unilateral, radiofrequency 50593 Ablation, renal tumor(s), unilateral, percutaneous, cryotherapy 50606 Endoluminal biopsy of ureter and/or renal pelvis, non-endoscopic, including imaging guidance (eg, ultrasound and/or fluoroscopy) and all associated radiological supervision and interpretation (List separately in addition to code for primary procedure) 50693 Placement of ureteral stent, percutaneous, including diagnostic nephrostogram and/or ureterogram when performed, imaging guidance (eg, ultrasound and/or fluoroscopy), and all associated radiological supervision and interpretation; pre-existing nephrostomy tract 50694 Placement of ureteral stent, percutaneous, including diagnostic nephrostogram and/or ureterogram when performed, imaging guidance (eg, ultrasound and/or fluoroscopy), and all associated radiological supervision and interpretation; new access, without separate nephrostomy catheter 50695 Placement of ureteral stent, percutaneous, including diagnostic nephrostogram and/or ureterogram when performed, imaging guidance (eg, ultrasound and/or fluoroscopy), and all associated radiological supervision and interpretation; new access, with separate nephrostomy catheter 50705 Ureteral embolization or occlusion, including imaging guidance (eg, ultrasound and/or fluoroscopy) and all associated radiological supervision and interpretation (List separately in addition to code for primary procedure) 50706 Balloon dilation, ureteral stricture, including imaging guidance (eg, ultrasound and/or fluoroscopy) and all associated radiological supervision and interpretation (List separately in addition to code for primary procedure) 57155 Insertion of uterine tandem and/or vaginal ovoids for clinical brachytherapy 66720 Ciliary body destruction; cryotherapy 69300 Otoplasty, protruding ear, with or without size reduction 77371 Radiation treatment delivery, stereotactic radiosurgery (SRS), complete course of treatment of cranial lesion(s) consisting of 1 session; multi-source Cobalt 60 based 77600 Hyperthermia, externally generated; superficial (ie, heating to a depth of 4 cm or less) 77605 Hyperthermia, externally generated; deep (ie, heating to depths greater than 4 cm) 77610 Hyperthermia generated by interstitial probe(s); 5 or fewer interstitial applicators 77615 Hyperthermia generated by interstitial probe(s); more than 5 interstitial applicators 92920 Percutaneous transluminal coronary angioplasty; single major coronary artery or branch 92921 Percutaneous transluminal coronary angioplasty; each additional branch of a major coronary artery (List separately in addition to code for primary procedure) 92924 Percutaneous transluminal coronary atherectomy, with coronary angioplasty when performed; single major coronary artery or branch 92925 Percutaneous transluminal coronary atherectomy, with coronary angioplasty when performed; each additional branch of a major coronary artery (List separately in addition to code for primary procedure) 92928 Percutaneous transcatheter placement of intracoronary stent(s), with coronary angioplasty when performed; single major coronary artery or branch 92929 Percutaneous transcatheter placement of intracoronary stent(s), with coronary angioplasty when performed; each additional branch of a major coronary artery (List separately in addition to code for primary procedure) 92933 Percutaneous transluminal coronary atherectomy, with intracoronary stent, with coronary angioplasty when performed; single major coronary artery or branch 92934 Percutaneous transluminal coronary atherectomy, with intracoronary stent, with coronary angioplasty when performed; each additional branch of a major coronary artery (List separately in addition to code for primary procedure) 92937 Percutaneous transluminal revascularization of or through coronary artery bypass graft (internal mammary, free arterial, venous), any combination of intracoronary stent, atherectomy and angioplasty, including distal protection when performed; single vessel 92938 Percutaneous transluminal revascularization of or through coronary artery bypass graft (internal mammary, free arterial, venous), any combination of intracoronary stent, atherectomy and angioplasty, including distal protection when performed; each additional branch subtended by the bypass graft (List separately in addition to code for primary procedure) 92941 Percutaneous transluminal revascularization of acute total/subtotal occlusion during acute myocardial infarction, coronary artery or coronary artery bypass graft, any combination of intracoronary stent, atherectomy and angioplasty, including aspiration thrombectomy when performed, single vessel 92943 Percutaneous transluminal revascularization of chronic total occlusion, coronary artery, coronary artery branch, or coronary artery bypass graft, any combination of intracoronary stent, atherectomy and angioplasty; single vessel 92944 Percutaneous transluminal revascularization of chronic total occlusion, coronary artery, coronary artery branch, or coronary artery bypass graft, any combination of intracoronary stent, atherectomy and angioplasty; each additional coronary artery, coronary artery branch, or bypass graft (List separately in addition to code for primary procedure) 92953 Temporary transcutaneous pacing 92960 Cardioversion, elective, electrical conversion of arrhythmia; external 92961 Cardioversion, elective, electrical conversion of arrhythmia; internal (separate procedure) 92973 Percutaneous transluminal coronary thrombectomy mechanical (List separately in addition to code for primary procedure) 92974 Transcatheter placement of radiation delivery device for subsequent coronary intravascular brachytherapy (List separately in addition to code for primary procedure) 92975 Thrombolysis, coronary; by intracoronary infusion, including selective coronary angiography 92986 Percutaneous balloon valvuloplasty; aortic valve 92987 Percutaneous balloon valvuloplasty; mitral valve 93312 Echocardiography, transesophageal, real-time with image documentation (2D) (with or without M-mode recording); including probe placement, image acquisition, interpretation and report 93313 Echocardiography, transesophageal, real-time with image documentation (2D) (with or without M-mode recording); placement of transesophageal probe only 93314 Echocardiography, transesophageal, real-time with image documentation (2D) (with or without M-mode recording); image acquisition, interpretation and report only 93315 Transesophageal echocardiography for congenital cardiac anomalies; including probe placement, image acquisition, interpretation and report 93316 Transesophageal echocardiography for congenital cardiac anomalies; placement of transesophageal probe only 93317 Transesophageal echocardiography for congenital cardiac anomalies; image acquisition, interpretation and report only 93318 Echocardiography, transesophageal (TEE) for monitoring purposes, including probe placement, real time 2-dimensional image acquisition and interpretation leading to ongoing (continuous) assessment of (dynamically changing) cardiac pumping function and to therapeutic measures on an immediate time basis 93451 Right heart catheterization including measurement(s) of oxygen saturation and cardiac output, when performed 93452 Left heart catheterization including intraprocedural injection(s) for left ventriculography, imaging supervision and interpretation, when performed 93453 Combined right and left heart catheterization including intraprocedural injection(s) for left ventriculography, imaging supervision and interpretation, when performed 93454 Catheter placement in coronary artery(s) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation 93455 Catheter placement in coronary artery(s) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation; with catheter placement(s) in bypass graft(s) (internal mammary, free arterial, venous grafts) including intraprocedural injection(s) for bypass graft angiography 93456 Catheter placement in coronary artery(s) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation; with right heart catheterization 93457 Catheter placement in coronary artery(s) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation; with catheter placement(s) in bypass graft(s) (internal mammary, free arterial, venous grafts) including intraprocedural injection(s) for bypass graft angiography and right heart catheterization 93458 Catheter placement in coronary artery(s) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation; with left heart catheterization including intraprocedural injection(s) for left ventriculography, when performed 93459 Catheter placement in coronary artery(s) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation; with left heart catheterization including intraprocedural injection(s) for left ventriculography, when performed, catheter placement(s) in bypass graft(s) (internal mammary, free arterial, venous grafts) with bypass graft angiography 2017 Moderate Conscious Sedation Changes (continue reading below) 93460 Catheter placement in coronary artery(s) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation; with right and left heart catheterization including intraprocedural injection(s) for left ventriculography, when performed 93461 Catheter placement in coronary artery(s) for coronary angiography, including intraprocedural injection(s) for coronary angiography, imaging supervision and interpretation; with right and left heart catheterization including intraprocedural injection(s) for left ventriculography, when performed, catheter placement(s) in bypass graft(s) (internal mammary, free arterial, venous grafts) with bypass graft angiography 93462 Left heart catheterization by transseptal puncture through intact septum or by transapical puncture (List separately in addition to code for primary procedure) 93463 Pharmacologic agent administration (eg, inhaled nitric oxide, intravenous infusion of nitroprusside, dobutamine, milrinone, or other agent) including assessing hemodynamic measurements before, during, after and repeat pharmacologic agent administration, when performed (List separately in addition to code for primary procedure) 93464 Physiologic exercise study (eg, bicycle or arm ergometry) including assessing hemodynamic measurements before and after (List separately in addition to code for primary procedure) 93505 Endomyocardial biopsy 93530 Right heart catheterization, for congenital cardiac anomalies 93561 Indicator dilution studies such as dye or thermodilution, including arterial and/or venous catheterization; with cardiac output measurement (separate procedure) 93562 Indicator dilution studies such as dye or thermodilution, including arterial and/or venous catheterization; subsequent measurement of cardiac output 93563 Injection procedure during cardiac catheterization including imaging supervision, interpretation, and report; for selective coronary angiography during congenital heart catheterization (List separately in addition to code for primary procedure) 93564 Injection procedure during cardiac catheterization including imaging supervision, interpretation, and report; for selective opacification of aortocoronary venous or arterial bypass graft(s) (eg, aortocoronary saphenous vein, free radial artery, or free mammary artery graft) to one or more coronary arteries and in situ arterial conduits (eg, internal mammary), whether native or used for bypass to one or more coronary arteries during congenital heart catheterization, when performed (List separately in addition to code for primary procedure) 93565 Injection procedure during cardiac catheterization including imaging supervision, interpretation, and report; for selective left ventricular or left atrial angiography (List separately in addition to code for primary procedure) 93566 Injection procedure during cardiac catheterization including imaging supervision, interpretation, and report; for selective right ventricular or right atrial angiography (List separately in addition to code for primary procedure) 93567 Injection procedure during cardiac catheterization including imaging supervision, interpretation, and report; for supravalvular aortography (List separately in addition to code for primary procedure) 93568 Injection procedure during cardiac catheterization including imaging supervision, interpretation, and report; for pulmonary angiography (List separately in addition to code for primary procedure) 93571 Intravascular Doppler velocity and/or pressure derived coronary flow reserve measurement (coronary vessel or graft) during coronary angiography including pharmacologically induced stress; initial vessel (List separately in addition to code for primary procedure) 93572 Intravascular Doppler velocity and/or pressure derived coronary flow reserve measurement (coronary vessel or graft) during coronary angiography including pharmacologically induced stress; each additional vessel (List separately in addition to code for primary procedure) 93582 Percutaneous transcatheter closure of patent ductus arteriosus 93583 Percutaneous transcatheter septal reduction therapy (eg, alcohol septal ablation) including temporary pacemaker insertion when performed 93609 Intraventricular and/or intra-atrial mapping of tachycardia site(s) with catheter manipulation to record from multiple sites to identify origin of tachycardia (List separately in addition to code for primary procedure) 93613 Intracardiac electrophysiologic 3-dimensional mapping (List separately in addition to code for primary procedure) 93615 Esophageal recording of atrial electrogram with or without ventricular electrogram(s) 93616 Esophageal recording of atrial electrogram with or without ventricular electrogram(s); with pacing 93618 Induction of arrhythmia by electrical pacing 93619 Comprehensive electrophysiologic evaluation with right atrial pacing and recording, right ventricular pacing and recording, His bundle recording, including insertion and repositioning of multiple electrode catheters, without induction or attempted induction of arrhythmia 93620 Comprehensive electrophysiologic evaluation including insertion and repositioning of multiple electrode catheters with induction or attempted induction of arrhythmia; with right atrial pacing and recording, right ventricular pacing and recording, His bundle recording 93621 Comprehensive electrophysiologic evaluation including insertion and repositioning of multiple electrode catheters with induction or attempted induction of arrhythmia; with left atrial pacing and recording from coronary sinus or left atrium (List separately in addition to code for primary procedure) 93622 Comprehensive electrophysiologic evaluation including insertion and repositioning of multiple electrode catheters with induction or attempted induction of arrhythmia; with left ventricular pacing and recording (List separately in addition to code for primary procedure) 93624 Electrophysiologic follow-up study with pacing and recording to test effectiveness of therapy, including induction or attempted induction of arrhythmia 2017 Moderate Conscious Sedation Changes (continue reading below) 93640 Electrophysiologic evaluation of single or dual chamber pacing cardioverter-defibrillator leads including defibrillation threshold evaluation (induction of arrhythmia, evaluation of sensing and pacing for arrhythmia termination) at time of initial implantation or replacement 93641 Electrophysiologic evaluation of single or dual chamber pacing cardioverter-defibrillator leads including defibrillation threshold evaluation (induction of arrhythmia, evaluation of sensing and pacing for arrhythmia termination) at time of initial implantation or replacement; with testing of single or dual chamber pacing cardioverter-defibrillator pulse generator 93642 Electrophysiologic evaluation of single or dual chamber transvenous pacing cardioverter-defibrillator (includes defibrillation threshold evaluation, induction of arrhythmia, evaluation of sensing and pacing for arrhythmia termination, and programming or reprogramming of sensing or therapeutic parameters) 93644 Electrophysiologic evaluation of subcutaneous implantable defibrillator (includes defibrillation threshold evaluation, induction of arrhythmia, evaluation of sensing for arrhythmia termination, and programming or reprogramming of sensing or therapeutic parameters) 93650 Intracardiac catheter ablation of atrioventricular node function, atrioventricular conduction for creation of complete heart block, with or without temporary pacemaker placement 93653 Comprehensive electrophysiologic evaluation including insertion and repositioning of multiple electrode catheters with induction or attempted induction of an arrhythmia with right atrial pacing and recording, right ventricular pacing and recording (when necessary), and His bundle recording (when necessary) with intracardiac catheter ablation of arrhythmogenic focus; with treatment of supraventricular tachycardia by ablation of fast or slow atrioventricular pathway, accessory atrioventricular connection, cavo-tricuspid isthmus or other single atrial focus or source of atrial re-entry 93654 Comprehensive electrophysiologic evaluation including insertion and repositioning of multiple electrode catheters with induction or attempted induction of an arrhythmia with right atrial pacing and recording, right ventricular pacing and recording (when necessary), and His bundle recording (when necessary) with intracardiac catheter ablation of arrhythmogenic focus; with treatment of ventricular tachycardia or focus of ventricular ectopy including intracardiac electrophysiologic 3D mapping, when performed, and left ventricular pacing and recording, when performed 93655 Intracardiac catheter ablation of a discrete mechanism of arrhythmia which is distinct from the primary ablated mechanism, including repeat diagnostic maneuvers, to treat a spontaneous or induced arrhythmia (List separately in addition to code for primary procedure) 93656 Comprehensive electrophysiologic evaluation including transseptal catheterizations, insertion and repositioning of multiple electrode catheters with induction or attempted induction of an arrhythmia including left or right atrial pacing/recording when necessary, right ventricular pacing/recording when necessary, and His bundle recording when necessary with intracardiac catheter ablation of atrial fibrillation by pulmonary vein isolation 93657 Additional linear or focal intracardiac catheter ablation of the left or right atrium for treatment of atrial fibrillation remaining after completion of pulmonary vein isolation (List separately in addition to code for primary procedure) 94011 Measurement of spirometric forced expiratory flows in an infant or child through 2 years of age 94012 Measurement of spirometric forced expiratory flows, before and after bronchodilator, in an infant or child through 2 years of age 94013 Measurement of lung volumes (ie, functional residual capacity [FRC], forced vital capacity [FVC], and expiratory reserve volume [ERV]) in an infant or child through 2 years of age Reference: 2017 CPT Codebook. CPT is a Trademark and owned by the American Medical Association |

ABOUT THE AUTHOR:
Ms. Pinky Maniri-Pescasio is the Founder of GoHealthcare Consulting. She is a National Speaker on Practice Reimbursement and a Physician Advocate. She has served the Medical Practice Industry for more than 25 years as a Professional Medical Practice Consultant. search hereArchives
July 2024
Categories
All
BROWSE HERE
All
|
|||||||||||||||
- About
- Leadership
- Contact Us
- Testimonials
- READ OUR BLOG
-
Let's Meet in Person
- 2023 ORTHOPEDIC VALUE BASED CARE CONFERENCE
- 2023 AAOS Annual Meeting of the American Academy of Orthopaedic Surgeons
- 2023 ASIPP 25th Annual Meeting of the American Society of Interventional Pain Management
- 2023 Becker's 20th Annual Spine, Orthopedic & Pain Management-Driven ASC Conference
- 2023 FSIPP Annual Conference by FSIPP FSPMR Florida Society Of Interventional Pain Physicians
- 2023 New York and New Jersey Pain Medicine Symposium
- Frequently Asked Questions and Answers - GoHealthcare Practice Solutions
- Readers Questions
Photos from shixart1985 (CC BY 2.0), www.ilmicrofono.it, shixart1985



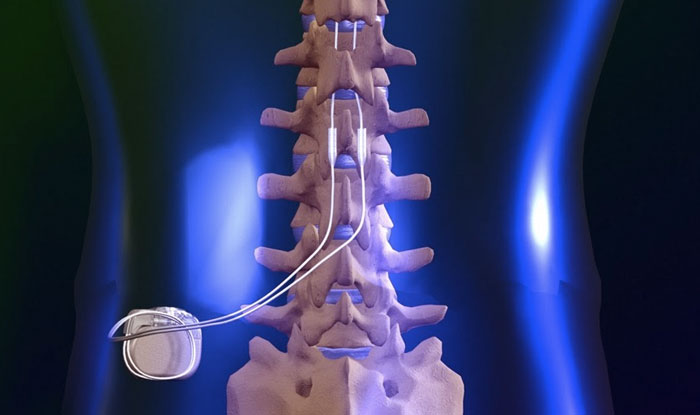


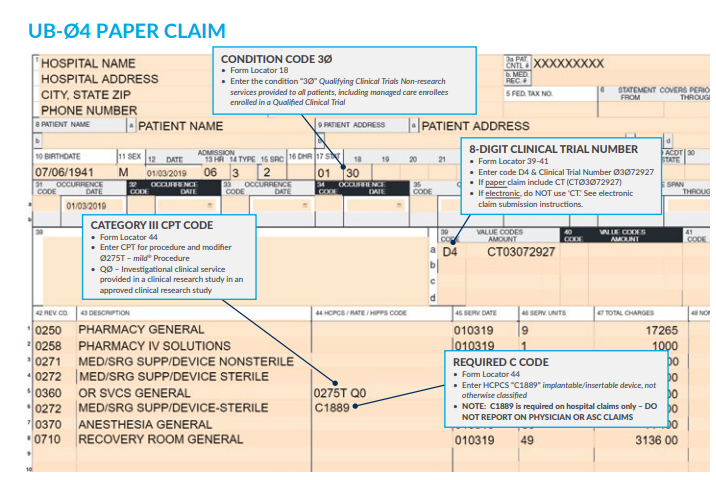

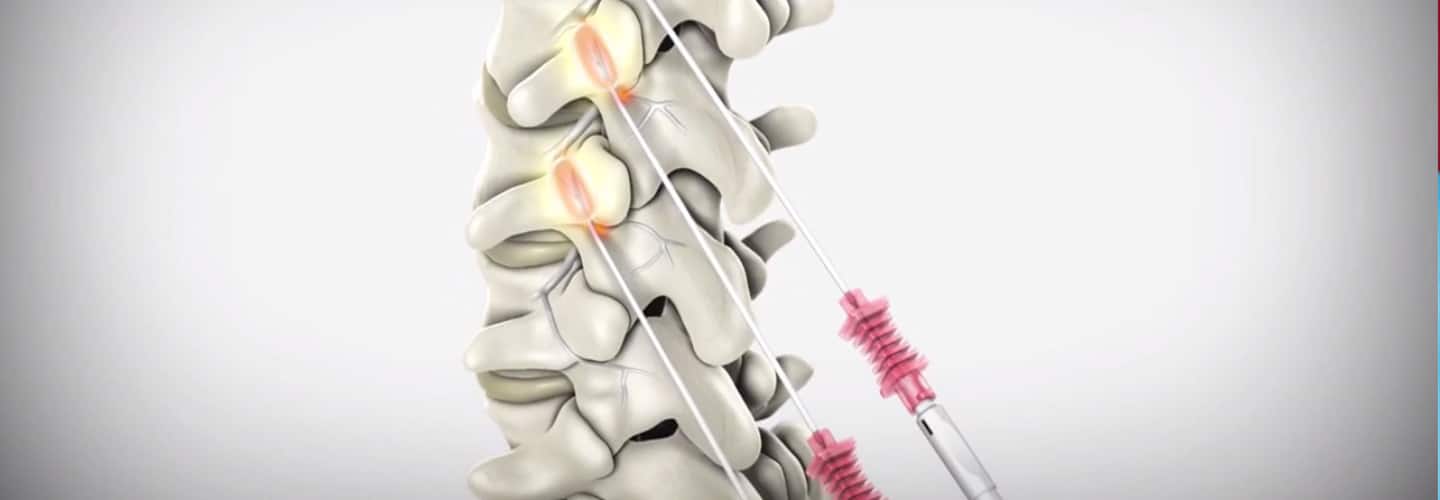

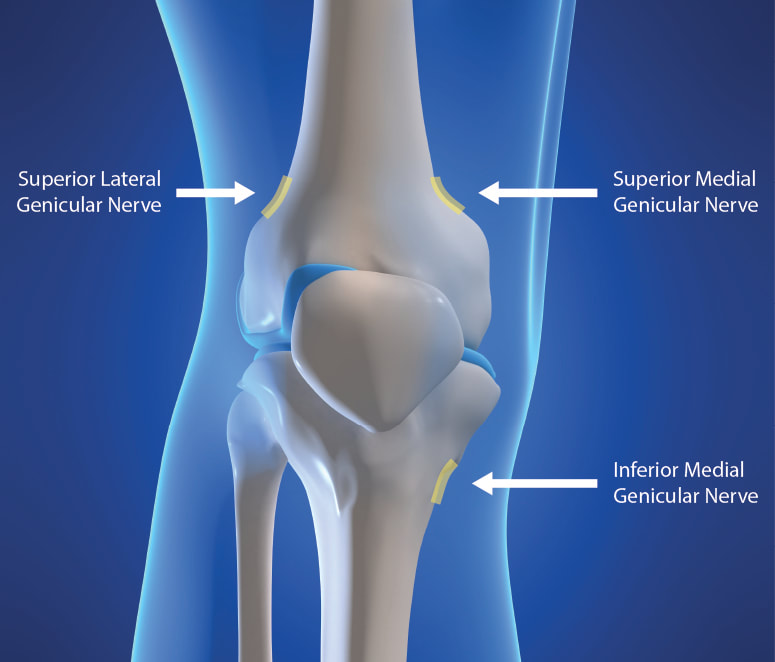
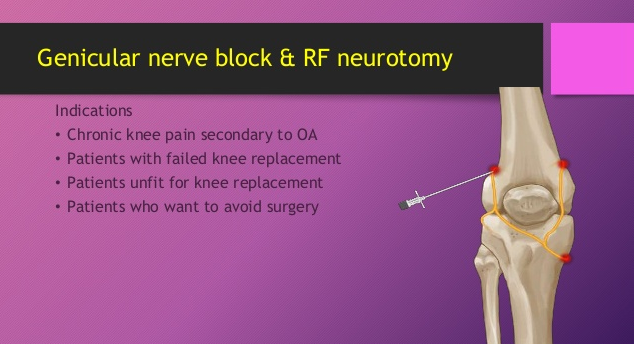

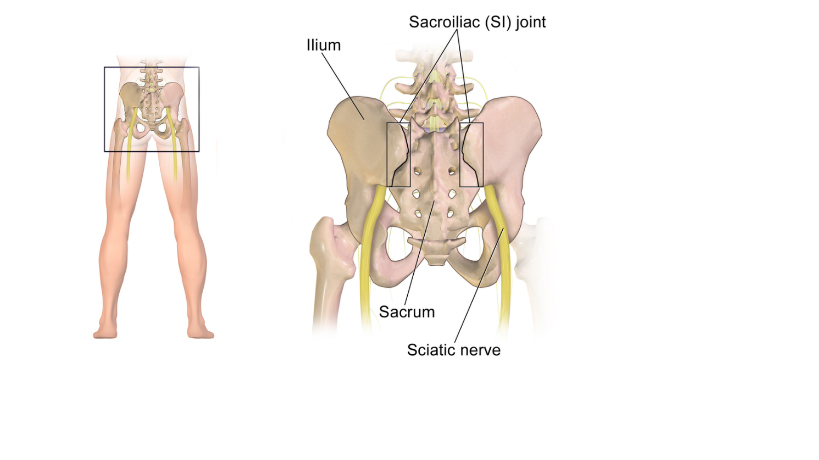
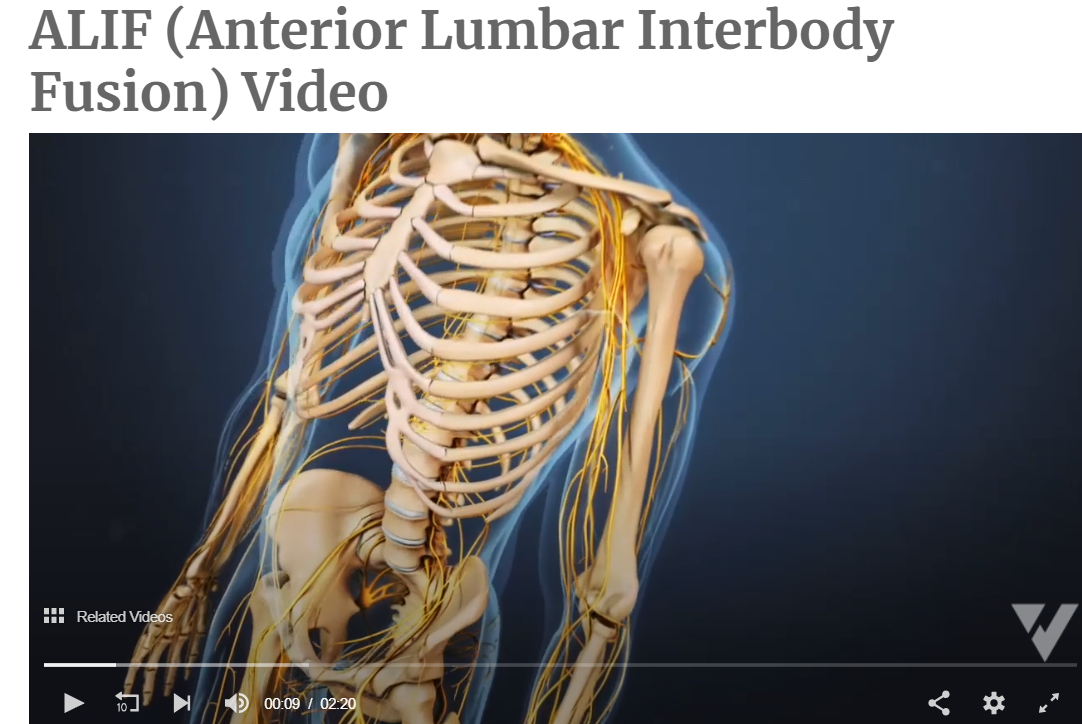
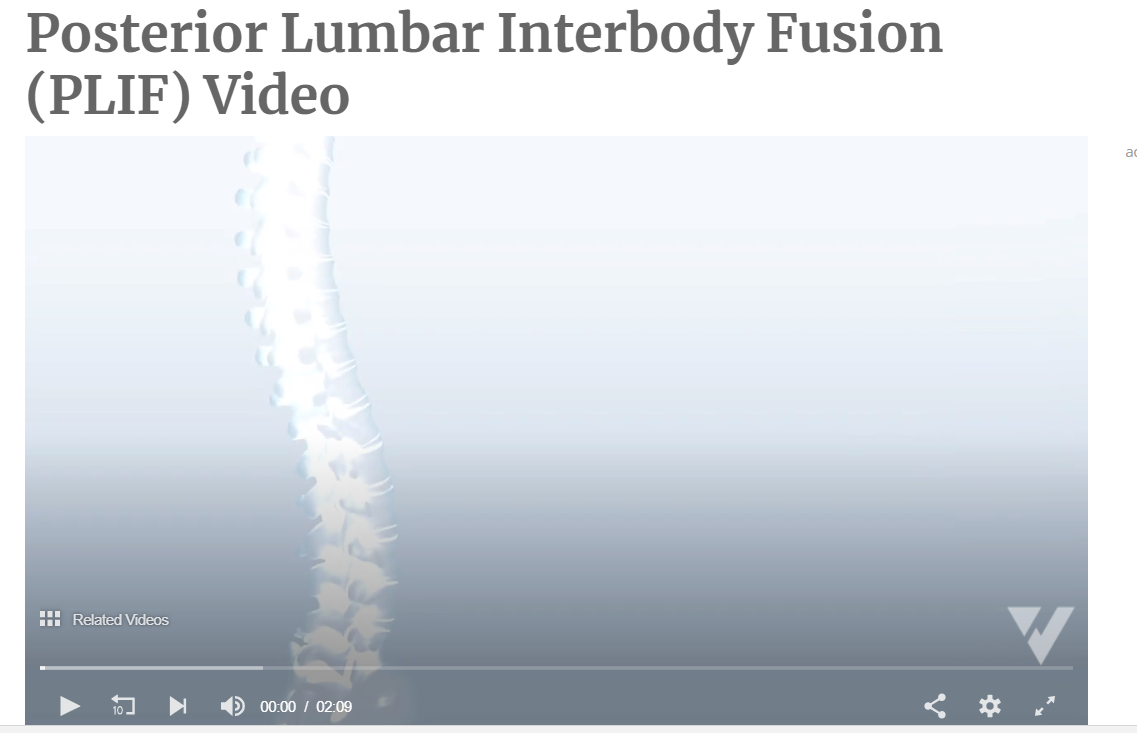
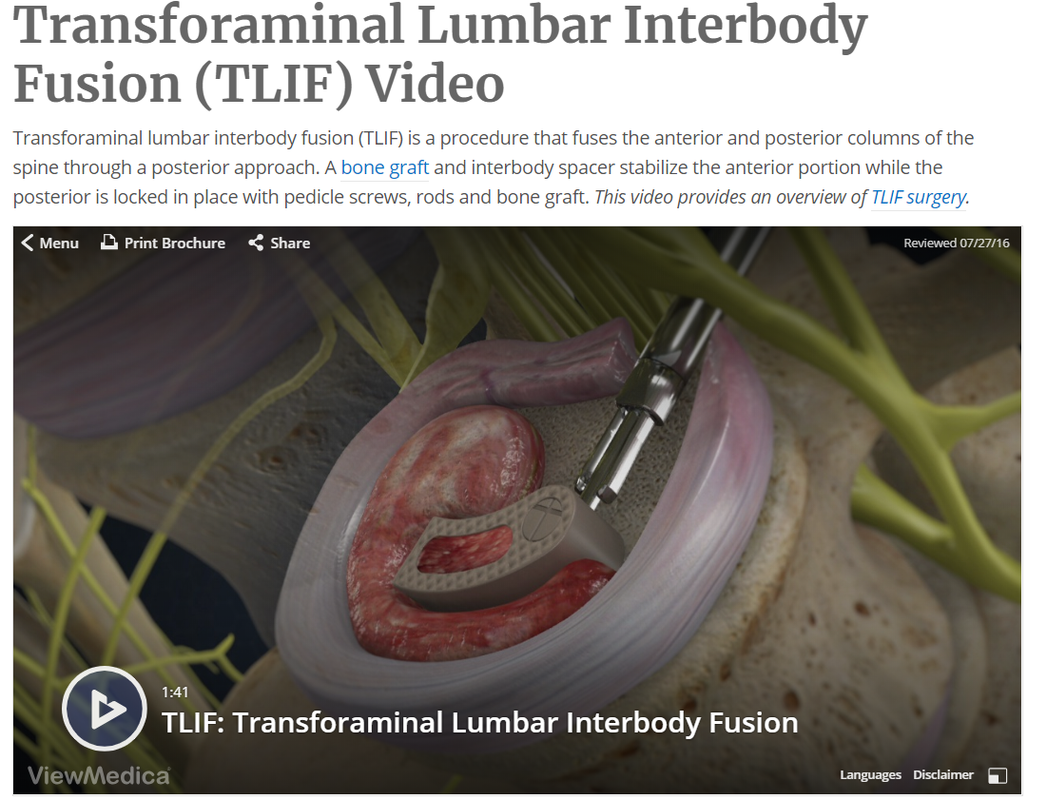

 RSS Feed
RSS Feed