|
Orthopedic surgeries are some of the most complex and delicate procedures in the field of medicine. In order to ensure that these procedures are carried out safely and efficiently, orthopedic surgeons require authorization from insurance companies. This process is known as prior authorization, and it can be time-consuming and cumbersome. That's why outsourcing prior authorization is becoming increasingly popular in the field of orthopedic surgery. In this article, we will discuss the importance of outsourcing orthopedic surgery prior authorization and how it can benefit both patients and healthcare providers. What is Prior Authorization?Prior authorization is a process used by insurance companies to determine whether they will cover the cost of a medical procedure or treatment. This process requires healthcare providers to submit detailed information about the procedure or treatment, including medical records, diagnosis codes, and treatment plans. Insurance companies review this information to determine whether the procedure or treatment is medically necessary and covered by the patient's insurance policy. The Importance of Prior Authorization for Orthopedic Surgery |
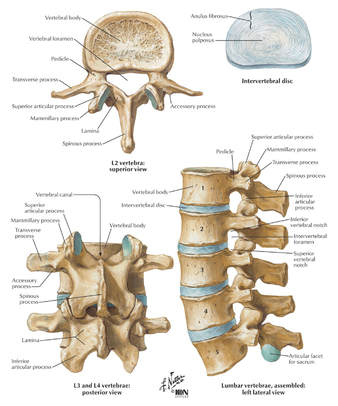
| Arthrodesis: Fusion, or permanent joining, of a joint, or point of union of two musculoskeletal structures, such as two bones. Disc/Intervertebral Disc: A round, flat fibrous tissue layer between two adjacent vertebrae consisting of a hard outer layer called annulus fibrosis and a jelly–like central part called nucleus pulposus; the intervertebral disc acts as a flexible cushion between the two vertebrae that helps in load bearing and shock absorption. Diskectomy: Excision of an intervertebral disk; also called discectomy. Fusion: Permanent joining. Graft: To repair or replace defective or missing tissue or structure with another material; repair of defects in body structures; artificial material, skin, part of a vessel, bone, or other tissue harvested and placed over or in a defect or used for anastomosis, i.e., to surgically connect two structures together. Herniation: Bulging of an organ or tissue through a weakness or opening in membranes or other structures. Intervertebral disk: Cushion of cartilage material separating two vertebrae. Laminectomy: Surgical excision of the lamina, one of the surfaces of a vertebra, one of the interlocking bones of the spine. Posterior interbody fusion: Spinal fusion with bone graft material placed in between the vertebrae. Posterior or posterolateral technique: Spinal fusion with bone graft material applied at the back or back and side of the vertebrae. Spinous process: Bony projection on the back of each vertebral bone. Spinal arthrodesis: Also called spinal fusion; a surgical procedure to join, or fuse, multiple vertebrae. | Annulus fibrosus: Fibrous outer ring of the intervertebral disk, the cartilage cushion between the interlocking bones in the spine; also known as the annular ring. Anterior interbody technique: Spinal fusion through an anterior, or front, approach, through the neck for cervical vertebrae, the chest for thoracic vertebrae, the abdomen for lumbar vertebrae. Bone grafting: Surgical procedure that replaces missing bone with material from the patient's own body, or from an artificial, synthetic, or natural substitute. Intervertebral disk: Cushion of cartilage material separating two vertebrae. Curette: Spoon shaped surgical instrument used for scraping; also spelled curet. Diskectomy: Excision of an intervertebral disk; also called discectomy. Lumbar spine: Lower back, containing vertebrae enumerated L1 through L5. Peritoneum: The membrane lining the abdominal cavity that attaches various organs to the abdominal wall. Presacral interbody technique: Spinal fusion procedure at L5 and S1 through an incision near the sacrum, or tailbone. Transverse process: A bony projection extending outward on each side of a vertebra. Stenosis: Narrowing of a vessel or other structure; a stenosed valve becomes stiff affecting its ability to open and close properly. Subcutaneous tissue: Tissue below the surface of the skin. Vertebra: One of the interlocking bones of the spine. Lamina: The flattened bone that extends from the vertebral pedicle medially and forms the posterior wall of the spinal foramen. |
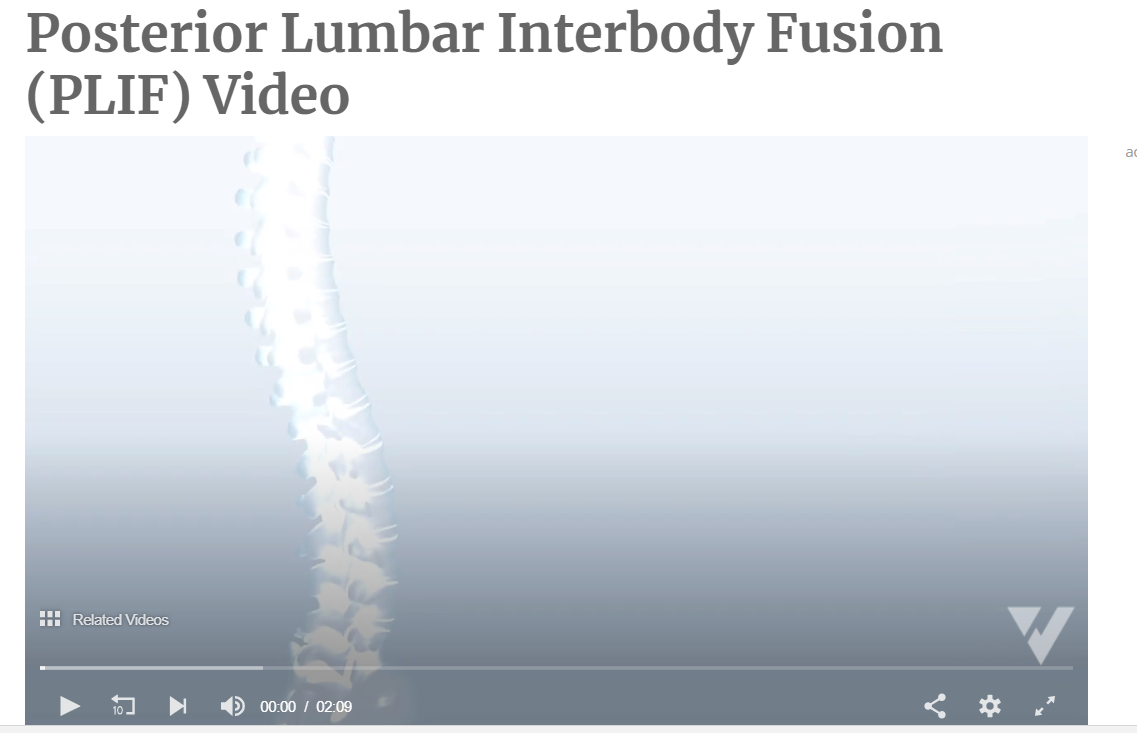
Understanding the Posterior Lumbar Interbody Spinal Fusion
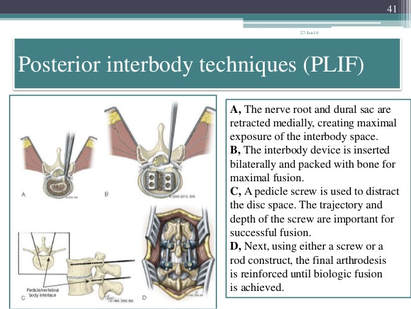
- Posterior Lumbar Interbody Fusion (PLIF) * Incision is performed through a midline incision in the back
Image Source: https://www.slideshare.net/drpraveenktripathi/lumbar-interbody-fusion-indications-techniques-and-complications
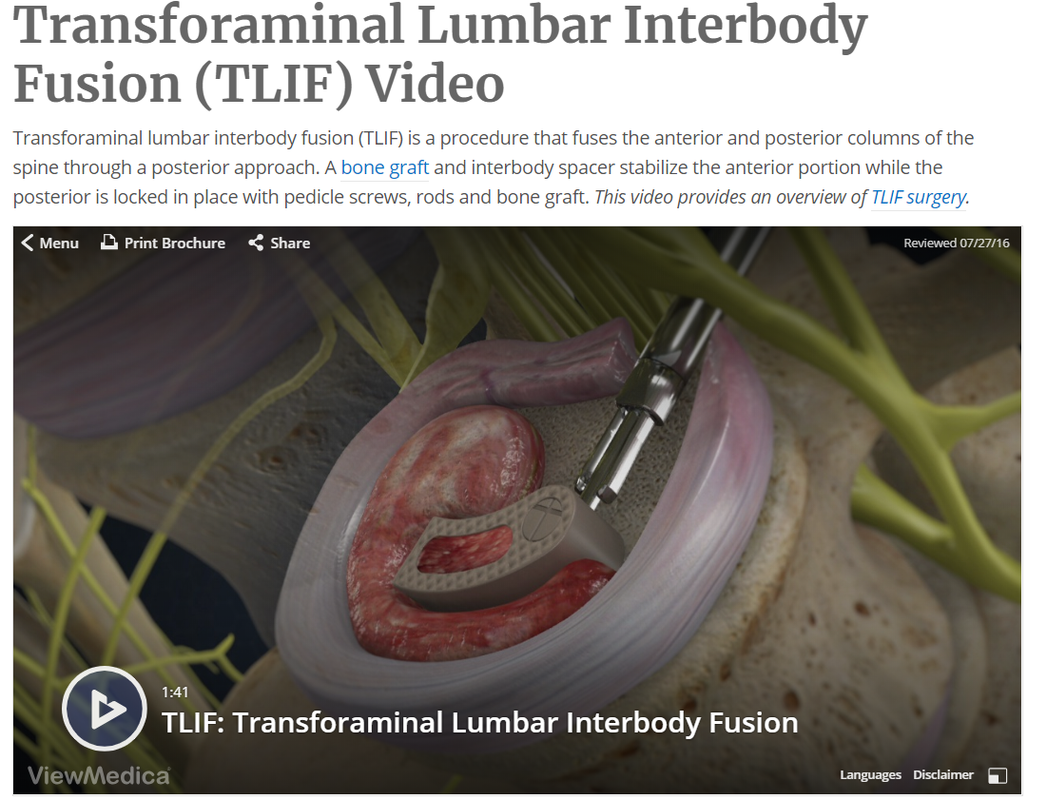
- Transforaminal Lumbar Interbody Fusion (TLIF) * Incision is also performed through a midline incision in the back, only on the side of the spine, to access the vertebral body at an angle
Your CPT® Codes for PLIF and TLIF Spinal Fusion Coding: CPT Code 22630, +22632
Arthrodesis, posterior interbody technique, including laminectomy and/or discectomy to prepare interspace (other than for decompression), single interspace; lumbar
+22632
Each Additional interspace (list separately in addition to code for primary procedure code)
Here's what occurs when 22630 is performed:
The provider performs an arthrodesis, also known as spinal fusion, in the lumbar spine, or lower back, to permanently join two vertebrae, the interlocking bones of the spine. He excises the lamina and disk material and applies bone graft between the disks to fuse them. The procedure helps to alleviate persistent pain caused by various spinal conditions, including herniated intervertebral disks, stenosis, or spinal injuries.
Then, in 2012
Code 22633 was introduced to to represent the combination of 22630 and 22612 Arthrodesis, posterior or posterolateral technique, single level; lumbar (with lateral transverse technique, when performed) at the same level.
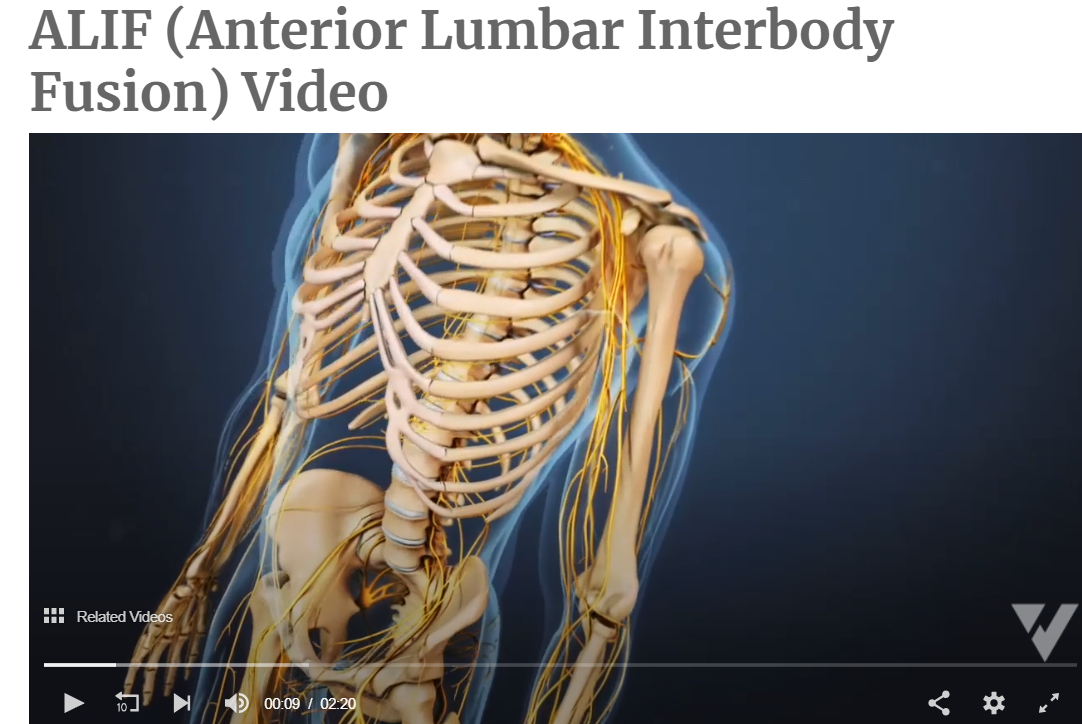
The Anterior Interbody Fusion Approach
| Techniques:
| |
Videos to watch for Procedure PLIF and TLIF
Your CPT® Codes for ALIF, DLIF and OLIF Spinal Fusion Coding: CPT Code 22558, +22585
Arthrodesis, anterior interbody technique, including minimal discectomy to prepare interspace (other than for decompression); lumbar
Remember: (For arthrodesis using pre-sacral interbody technique, see 22586, 0195T)
+22585
Arthrodesis, anterior interbody technique, including minimal discectomy to prepare interspace (other than for decompression); each additional interspace (List separately in addition to code for primary procedure)
Remember: (Use 22585 in conjunction with 22554, 22556, 22558)
(Do not report 22585 in conjunction with 63075, even if performed by a separate individual. To report anterior cervical discectomy and interbody fusion at the same level during the same session, use 22552)
Here's what occurs when 22558 is being performed:
The provider performs arthrodesis, also known as spinal fusion, in the lower back, to permanently join two vertebrae, the interlocking bones of the spine, to alleviate persistent pain caused by a herniated, or bulging, disk, or other spinal condition. He makes an incision in the abdomen to access the spine and remove disk material.
Instrumentation may be required to stabilize the Spinal Fusion
Add-Code +22840 Posterior non-segmental instrumentation (eg, Harrington rod technique, pedicle fixation across 1 interspace, atlantoaxial transarticular screw fixation, sublaminar wiring at C1, facet screw fixation) (List separately in addition to code for primary procedure)
(Use 22840 in conjunction with 22100-22102, 22110-22114, 22206, 22207, 22210-22214, 22220-22224, 22310-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040-63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081, 63085, 63087, 63090, 63101, 63102, 63170-63290, 63300-63307)
Add-On Code +22841 Internal spinal fixation by wiring of spinous processes (List separately in addition to code for primary procedure)
Add-On Code +22842 Posterior segmental instrumentation (eg, pedicle fixation, dual rods with multiple hooks and sublaminar wires); 3 to 6 vertebral segments (List separately in addition to code for primary procedure)
Use 22842 in conjunction with 22100- 22102, 22110- 22114, 22206, 22207, 22210- 22214, 22220-22224, 22305-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040-63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081, 63085, 63087, 63090, 63101, 63102, 63170-63290, 63300- 63307)Text has been updated
Add-On Code +22843 7 to 12 vertebral segments (List separately in addition to code for primary procedure)
(Use 22843 in conjunction with 22100- 22102, 22110-22114, 22206, 22207, 22210- 22214, 22220-22224, 22305-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040-63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081,63085, 63087, 63090, 63101, 63102, 63170-63290,63300- 63307)Text has been updated
Add-On Code +22844 13 or more vertebral segments (List separately in addition to code for primary procedure)
(Use 22844 in conjunction with 22100- 22102, 22110-22114, 22206, 22207, 22210- 22214, 22220-22224, 22305-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040-63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081, 63085, 63087, 63090, 63101, 63102, 63170-63290, 63300- 63307)
ANTERIOR INSTRUMENTATION
Add-On Code +22845 Anterior instrumentation; 2 to 3 vertebral segments (List separately in addition to code for primary procedure)
(Use 22845 in conjunction with 22100- 22102, 22110-22114, 22206, 22207, 22210- 22214, 22220-22224, 22305-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040-63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081,63085, 63087, 63090, 63101, 63102, 63170-63290,63300- 63307)Text has been updated
Add-On Code +22846 4 to 7 vertebral segments (List separately in addition to code for primary procedure)
Use 22846 in conjunction with 22100- 22102, 22110-22114, 22206, 22207, 22210- 22214, 22220-22224, 22305-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040-63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081, 63085, 63087, 63090, 63101, 63102, 63170-63290, 63300- 63307)Text has been updated
Add-On Code +22847 8 or more vertebral segments (List separately in addition to code for primary procedure)
(Use 22847 in conjunction with 22100-22102, 22110-22114, 22206, 22207, 22210-22214, 22220-22224, 22305-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040- 63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081, 63085, 63087, 63090, 63101, 63102, 63170-63290,63300-63307)Text has been updated
Add-On Code +22848 Pelvic fixation (attachment of caudal end of instrumentation to pelvic bony structures) other than sacrum (List separately in addition to code for primary procedure)
(Use 22848 in conjunction with 22100- 22102, 22110-22114, 22206, 22207, 22210- 22214, 22220-22224, 22305-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040-63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081, 63085, 63087, 63090, 63101, 63102, 63170-63290, 63300- 63307)

Spinal Prosthetic Devices may also be required to be reported CPT Code 22853
Insertion of interbody biomechanical device(s) (eg, synthetic cage, mesh) with integral anterior instrumentation for device anchoring (eg, screws, flanges), when performed, to intervertebral disc space in conjunction with interbody arthrodesis, each interspace (List separately in addition to code for primary procedure)
Notes:
(Use 22853 in conjunction with 22100-22102, 22110-22114, 22206, 22207, 22210-22214, 22220-22224, 22310-22327, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812, 63001-63030, 63040-63042, 63045-63047, 63050-63056, 63064, 63075, 63077, 63081, 63085, 63087, 63090, 63101, 63102, 63170-63290, 63300-63307)
(Report 22853 for each treated intervertebral disc space)
Code +22853 is one of several new codes within the spine section for the insertion of biomechanical devices that replace deleted code +22851 (Application of intervertebral biomechanical device[s] ...). The new add-on codes are more specific regarding the type and location of the biomechanical devices.
CPT® guidelines direct you to report +22853 for each treated intervertebral disc space.
Report +22853 in addition to the definitive procedure(s) since it is an add-on code.
Do not append modifier 62 (Two surgeons) to 22853.
The provider inserts a metallic cage or mesh device between two vertebrae and may use screws or flanges to attach it to the front part of the vertebrae; the device maintains the disc space, provides spinal stability, and yet preserves some range of motion, which helps relieve persistent pain caused by a herniated, or bulging, disk or other spinal condition. The provider performs this procedure during a spinal interbody arthrodesis procedure, which is fusion, or permanent joining, of vertebrae over the joint space.
Remember!
Code +22853 is an add–on code and must be reported with an appropriate primary procedure, such as 22548–22586 (Anterior or anterolateral approach technique arthrodesis procedures on the spine [vertebral column]), but there are many other codes that can be reported as a primary code.
Report one unit of this code for each interspace treated, not for the number of devices inserted. For example, if the provider inserts two cages into a single interspace, you report this code only once. If the provider inserts a device at two separate interspaces, e.g., between C3–4 and C5–6, then you would report this code twice.
This code is for the application of a device to expand or maintain an intervertebral disc space.
For a similar procedure to cover a defect created by removal of a vertebral body,
report 22854 (Insertion of intervertebral biomechanical device(s) [e.g., synthetic cage, mesh] with integral anterior instrumentation for device anchoring [e.g., screws, flanges], when performed, to vertebral corpectomy[ies] [vertebral body resection, partial or complete] defect, in conjunction with interbody arthrodesis, each contiguous defect [List separately in addition to code for primary procedure]).
For insertion of a similar device to treat an intervertebral disc space or vertebral body removal defect but without interbody fusion (arthrodesis), report 22859 (Insertion of intervertebral biomechanical device[s] [e.g., synthetic cage, mesh, methylmethacrylate] to intervertebral disc space or vertebral body defect without interbody arthrodesis, each contiguous defect [List separately in addition to code for primary procedure]).
Report Bone Grafting if allowable, CPT Code 20930
Allograft, morselized, or placement of osteopromotive material, for spine surgery only (List separately in addition to code for primary procedure)
Notes:
(Use 20930 in conjunction with 22319, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812)
Here's what occurs when 20930 is being performed;
The provider applies small pieces of donor or synthetic bone graft material during a spinal surgery to encourage bone growth during the healing period.
Coding Tip!
Code 20930 is an add on code and used for specified spinal procedures only.
Check with your payer to determine if 20930 can be billed separately or if the application of the bone graft material is included in the code for the primary surgical procedure.
Do not append modifier 62 to bone graft codes 20900-20938.
(For spinal surgery bone graft[s] see codes 20930-20938)
Check with your payer if you can separately report this code;
+20931
Allograft, structural, for spine surgery only (List separately in addition to code for primary procedure)
Notes:
(Use 20931 in conjunction with 22319, 22532-22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812)
A provider uses a structural allograft, a type of donor bone, to fill in bony defects as she performs a spinal surgery procedure.
Coding Tips:
Code 20931 is an add on code describing application of structural allograft to spinal defects and must be reported with an allowable primary spinal procedure code.
Report 20930, Allograft, morselized, or placement of osteopromotive material, for spine surgery only, together with 20931 only in the case of a human donor who is a different person from the recipient.
You should never append modifier 50, Bilateral procedure, to 20931. The CMS Physician Fee Schedule Database includes a 9 indictor in the BILAT SURG column for this code. According to further CMS instructions, a 9 indicator in this column means that the concept of a bilateral surgery with spinal grafting does not apply.
+20936
Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process, or laminar fragments) obtained from same incision (List separately in addition to code for primary procedure)
Notes:
(Use 20936 in conjunction with 22319, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812)
A provider uses an autograft, a type of donor bone, to fill in bony defects as she performs a spinal surgery procedure. She extracts the autograft from the patient’s own bone, taken from the same surgical incision.
Coding Tips:
Code 20936 is an add on code describing grafting from a donor area using the same incision during a major operative procedure and must be reported with an allowable primary spinal procedure code.
You should never append modifier 50, Bilateral procedure, to 20936. The CMS Physician Fee Schedule Database includes a 9 indictor in the BILAT SURG column for this code. According to further CMS instructions, a 9 indicator in this column means that the concept of a bilateral surgery with spinal grafting does not apply.
+20937
Autograft for spine surgery only (includes harvesting the graft); morselized (through separate skin or fascial incision) (List separately in addition to code for primary procedure)
Notes:
(Use 20937 in conjunction with 22319, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812)
The provider uses an autograft, a type of donor bone, to fill in bony defects as she performs a spinal surgery procedure. She extracts the autograft from the patient’s own body during the surgical procedure, through a separate incision.
Coding Tips:
Code 20937 is an add on code describing preparation and application of a morselized autograft through a separate skin incision and must be reported with an allowable primary spinal procedure code.
*** A vertebral segment describes the basic constituent part into which the spine may be divided. It represents a single complete vertebral bone with its associated articular processes and laminae. A vertebral interspace is the non-bony compartment between two adjacent vertebral bodies which contains the intervertebral disc, and includes the nucleus pulposus, annulus fibrosus, and two cartilaginous endplates.
Autograft for spine surgery only (includes harvesting the graft); structural, bicortical or tricortical (through separate skin or fascial incision) (List separately in addition to code for primary procedure)
Notes:
(Use 20938 in conjunction with 22319, 22532, 22533, 22548-22558, 22590-22612, 22630, 22633, 22634, 22800-22812)
(For aspiration of bone marrow for bone grafting, spine surgery only, use 20939)
The provider uses an autograft, a type of donor bone, to fill in bony defects as she performs a spinal surgery procedure. She extracts the autograft from the patient's own body during the surgical procedure, through a separate incision.
Reporting Cosurgeries
Coding Tip
Reporting Cosurgeries
"We receive many questions concerning how to report surgeries performed by more than one physician. To help you understand the proper coding we present the following information."
The General Question
"I am a general surgeon who sometimes performs surgeries with other surgeons (cosurgeries), such as orthopedic or neurosurgeons. I open the surgical site, the other surgeon does the definitive portion of the procedure, and then I close. What CPT codes should I report for my services? I have heard from some sources that I should bill for a thoracotomy and wound repair. But other sources have told me to report the same CPT codes as the other surgeon. Which is correct?
CPT® ASSISTANT'S REPLY:
Here's How to Code:
"For situations in which one surgeon performs the opening and closing of a surgery and another physician performs the definitive portion of the procedure, both physicians should report the same CPT codes, and appropriately append either modifier -62 or modifier -66."
Illustration
A patient's surgery includes arthrodesis of two interspaces of the thoracic spine by anterior interbody technique, with anterior instrumentation of three vertebral segments. Physician "A" performs a thoracotomy at the start of the surgical session, and Physician "B" performs the arthrodesis and spinal instrumentation. Upon completion of the arthrodesis and spinal instrumentation, Physician A closes the operative site.
Coding the Illustration
(The physicians in the illustration would report the codes indicated below.)
Physician A 22556-62
Physician B 22556-62
22558-62
22558-62
22845-62
22845-62
When performing these cosurgeries, it is important to communicate with the other surgeon's office to be certain that you submit the claims properly
Coding Communication
How to Code Prosthetic Devices
It is not often that we devote an entire article to a single code, but sometimes this is the only way to fully explain the use of certain codes-22851, application of prosthetic device (eg, metal cages, methylmethacrylate) to vertebral defect or interspace, is such a code. But before we review how to report this code, it is probably a good idea to first do a brief anatomical review of the vertebral column.
The vertebral column (spine) consists of a series of bones known as vertebrae.
An adult human possesses 33 vertebrae divided into the following five types:
7 cervical vertebrae;
12 thoracic vertebrae;
5 lumbar vertebrae;
5 sacral vertebrae; and
4 coccygeal vertebrae.
The sacral vertebrae are typically fused into a single bone known as the sacrum. The coccygeal vertebrae are sometimes fused into a single bone known as the coccyx.
Therefore, the actual number of bones in the vertebral column may be 26-29, depending on if the coccygeal vertebrae are fused. Vertebrae are commonly named by a letter that corresponds to the region of the vertebral column to which the vertebrae belongs, followed by a number that indicates where in the region the vertebrae is located. For example, the most superior cervical vertebra is called C1, with the next cervical vertebrae down designated C2. The most superior thoracic vertebrae is T1, with the next one down designated T2.
Fig. 1 - Spinal Prosthetic Devices
Between each pair of vertebrae is a disc that cushions the spinal column. If one of the discs degenerates or if one of the 26-29 vertebrae are injured (as in the case of a fracture, degenerative disease, or secondary to tumor destruction) the physician may need to place a prosthetic device (eg, metal cages or methyl-methacrylate) in the vertebral defect or interspace. (Fig. 1) In these instances, a segment of vertebral level may be drilled and metal cages packed with porous implants of bone graft may be inserted or methylmethacrylate may be placed between the affected vertebrae.
Proper Reporting of code 22851
It is important to note that CPT® code 22851 is not intended to be reported per cage.
CPT® code 22851 should only be reported one time, regardless if one or more metal cages are placed in the intervertebral space at the same level. However, if metal cages are placed at two different levels, (eg, metal cage placed at L3-4 interspace and L5-S1 interspace), then 22851 may be reported more than once to indicate that one or more cages were placed at two or more different levels. It is important to note that a single cage or methylmethacrylate can cover a defect of several vertebral segments (eg, a single cage may replace three entire vertebrae), wherein code 22851 would still only be reported one time.
Within the spine section, instrumentation procedure codes (22840-22855) are reported in addition to the definitive procedure(s) without appending the modifier -51. Therefore, if arthrodesis is performed in addition to the placement of the metal cages, then it would be appropriate to report code 22851 in addition to the appropriate arthrodesis code, 22548-22632. In this instance, the modifier -51 would not be appended to code 22851.
If metal cages are placed through an anterior approach and pedicle screws are placed through a posterior approach, it would be appropriate to report both code 22851 and one of the codes from the posterior instrumentation series, 22840, 22842-22844. However, if different instrumentation is used in addition to the metal cages or methylmethacrylate through the same approach (eg, an anterior plating system) or pedicle screws and posterior lumbar interbody fusion utilizing cages), then the appropriate instrumentation code would be reported in addition to code 22851. However, 22851 and 22845 should not both be reported if only the metal cage is inserted.
If fracture treatment, dislocation, or arthrodesis is performed in addition to spinal instrumentation, then the appropriate fracture treatment, dislocation or arthrodesis code (22325, 22326, 22327, 22548-22812) would be reported separately in addition to code 22851. In this instance, CPT® code 22851 would be reported in addition to the definitive procedure(s) without the modifier -51 appended.
If bone grafting is performed in addition to code 22851, then the appropriate bone grafting code, 20930-20938, would be reported additionally.
Clinical Sample:
CPT® Code 22851
A 50-year-old man undergoes an anterior fusion of L5-S1 for degenerative disease. A retroperitoneal incision is made and an arthrodesis performed using a BAK cage. A distracter is placed in the interspace, a hole is drilled in the interspace, and the BAK cage is placed in the hole. The spacer is removed and replaced with another BAK cage. Both cages are filled with bone graft. (Report arthrodesis and/or bone grafting separately using the appropriate CPT code[s]).
The exposed disk space and adjacent vertebrae are prepared with bone-cutting instruments for acceptance of the prosthetic device. Preparation of the recipient site is made according to the protocol of the particular device. If methylmethacrylate is to be used, a screw or pin may be inserted into the adjacent vertebral surfaces to anchor the methylmethacrylate. Provision is made for cooling of adjacent tissues and protection of heat sensitive tissue from the exothermic reaction of the curing of the methylmethacrylate. For cages, the recipient site is prepared by bone dissection, a trial fit with the device or a spacer or template as indicated by the protocol is inserted and removed for any final modifications of the recipient site. The prosthetic device is then screwed, impacted, or injected into place according to protocol for this particular device. (Additional fixation, other provision for arthrodesis, or bone grafting are coordinated with the placement of the prosthetic device and are coded separately.) For devices that incorporate graft material, that material is appropriately placed into the device prior to its final insertion.
CPT® ASSISTANT September 2000 page 10
Coding Consultation
Musculoskeletal System, Surgery, 22548-22585, 22899 (Q&A)
Question
"Should I use the anterior or anterolateral approach technique arthrodesis series of codes (22548-22585) to report intra-abdominal laparoscopic, video assisted anterior interbody fusion?"
AMA CPT® Comment
"The anterior or anterolateral approach technique arthrodesis series of codes (22548-22585) are intended to describe arthrodesis performed via an open surgical approach. There is not a specific CPT code that accurately describes laparoscopic anterior interbody fusion. Therefore, code 22899, Unlisted procedure, spine should be reported. When reporting an unlisted code to describe a procedure or service, it will be necessary to submit supporting documentation (eg, procedure report) along with the claim to provide an adequate description of the nature, extent, need for the procedure, and the time limit, effort, and equipment necessary to provide the service."
CPT® ASSISTANT March 2015 page 9
Frequently Asked Questions:Surgery: Musculoskeletal System
Question: "Are CPT codes 22851 and 22845 appropriate to report when modular implants, such as the RSB (RSB LLC; Cleveland, OH) InterPlate® (a modular interbody platform technology), are implanted for spinal fusion procedures?"
Answer:
"No. The RSB InterPlate® describes a stand-alone interbody fusion device that consists of an interbody spacer with screw fixation or other mechanisms, which engage adjacent vertebrae. Such devices should be reported with code 22558, Arthrodesis, anterior interbody technique, including minimal discectomy to prepare interspace (other than for decompression); lumbar, and 22851, Application of intervertebral biomechanical device(s) (eg, synthetic cage(s), methylmethacrylate) to vertebral defect or interspace (List separately in addition to code for primary procedure). An additional anterior instrumentation code (22845) is not applicable because there is no separate construct placed across the vertebral segment."
Question: "Would it be appropriate to separately report any of the following with the hammertoe correction code 28285 (2nd digit), if adequately documented? (1) Resection of hypertrophied base of proximal phalanx (28126), if performed through a separate incision at the metatarsophalangeal (MTP joint); (2) flexor tenotomy (28232) performed through a separate incision at the distal interphalangeal (DIP) joint; (3) an additional unit of 28285 if K-wire is inserted through the DIP, MTP, or proximal interphalangeal (PIP) joint."
Answer:
"No. Code 28126, Resection, partial or complete, phalangeal base, each toe; code 28232, Tenotomy, open, tendon flexor; toe, single tendon (separate procedure); and the insertion of K-wire through DIP, PIP, and MTP joints are all inclusive components of the procedure described by code 28285, Correction, hammertoe (eg, interphalangeal fusion, partial or total phalangectomy), and should not be reported separately."
2020 AMA's CPT® Guidelines
2019 AMA's CPT® Guidelines
2018 AMA's CPT® Guidelines
2017 AMA's CPT® Guidelines
CPT® Assistant Archives
Websites:
NASS
Spine-Health
Medtronic
Ahima
AAPC
CMS
All other commercial payers clinical guidelines from the public domains on the internet
Read more blog posts:
Commonly Use DME Modifiers | Durable Medical Equipment Billing
- Make sure you have identified medical necessity for a DME Durable Equipment Item
- Make sure you have documented the necessity
- Make sure you have a delivery receipt that the patient had received the DME Item
- Make sure you are reporting the right DME Item
- Make sure you are reporting the right place of service
- Make sure you have checked with your payer how they want you to report the DME Item on your Claims
This modifier is used for DME items that are rented, and will be used for equipment in the following categories:
- Inexpensive or other Routinely purchased DME (IRP)
- Frequent or Substantial Servicing (FS)
- Certain customized items:
- Other Prosthetic and Orthotic Devices (P & O)
- Capped Rental Items (CR)
- Oxygen and Oxygen Equipment
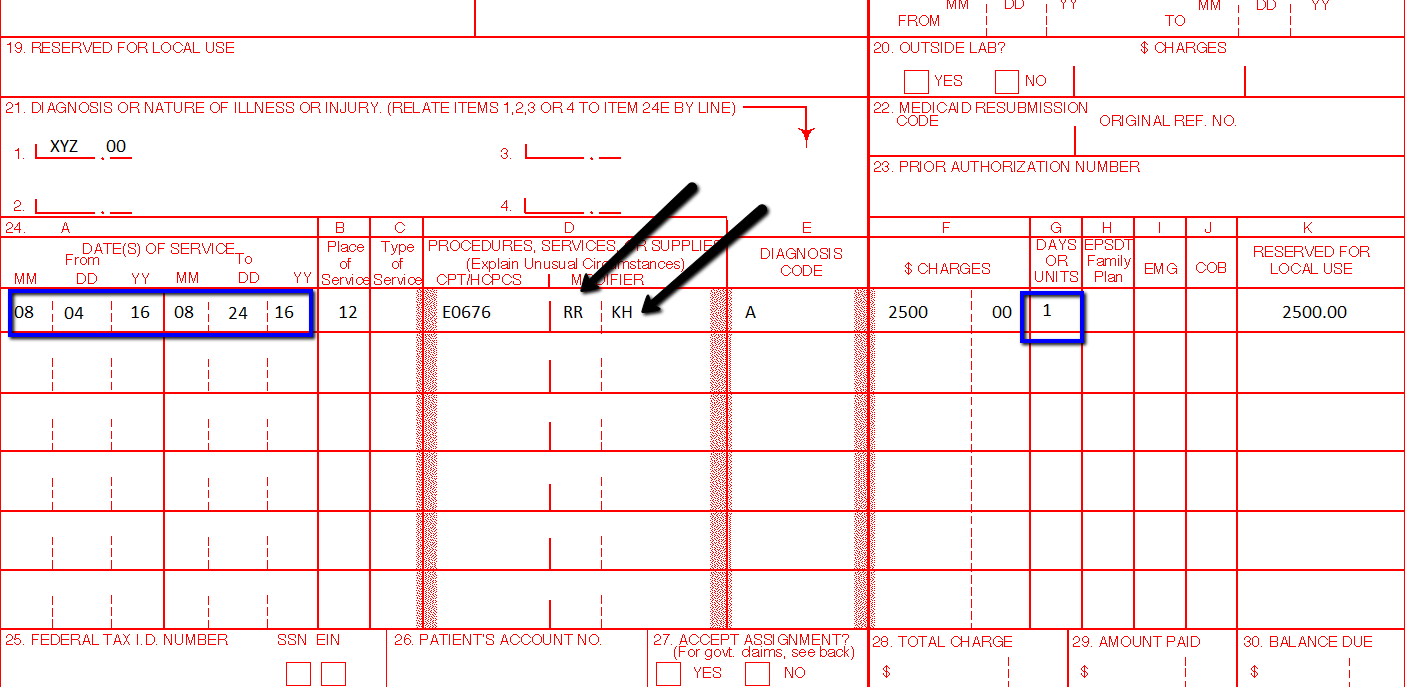
KH — DMEPOS ITEM, INITIAL CLAIM, PURCHASE OR FIRST MONTH RENTAL
This modifier is used for a capped rental DME item. When using the KH modifier, you are indicating you are billing for the first month of the capped rental period.
KJ — DMEPOS ITEM, PARENTERAL ENTERAL NUTRITION (PEN) PUMP OR CAPPED RENTAL, Month four to fifteen. This modifier is used for capped rental DME items. When using the KJ modifier, you are indicating you are billing for months four through thirteen/fifteen of the capped rental period.
KI — DMEPOS ITEM, SECOND OR THIRD MONTH RENTAL
This modifier is used for capped rental DME items. When using the KI modifier, you are indicating you are billing for the second and/or third month of the capped rental period
A8 — DRESSING FOR EIGHT WOUNDS. (EFFECTIVE DATE 1/1/2003)
Surgical dressing codes billed without modifiers A1-A9 (see Coding Guidelines) are noncovered under the Surgical Dressings benefit. Certain dressings may be covered under other benefits.
A5 — DRESSING FOR FIVE WOUNDS. (EFFECTIVE DATE 1/1/2003)
Surgical dressing codes billed without modifiers A1-A9 (see Coding Guidelines) are noncovered under the Surgical Dressings benefit. Certain dressings may be covered under other benefits.
A4 — DRESSING FOR FOUR WOUNDS. (EFFECTIVE DATE 1/1/2003)
Surgical dressing codes billed without modifiers A1-A9 (see Coding Guidelines) are noncovered under the Surgical Dressings benefit. Certain dressings may be covered under other benefits.
A9 — DRESSING FOR NINE OR MORE WOUNDS. (EFFECTIVE DATE 1/1/2003)
Surgical dressing codes billed without modifiers A1-A9 (see Coding Guidelines) are noncovered under the Surgical Dressings benefit. Certain dressings may be covered under other benefits.
A1 — DRESSING FOR ONE WOUND. (EFFECTIVE DATE 1/1/2003)
Surgical dressing codes billed without modifiers A1-A9 (see Coding Guidelines) are noncovered under the Surgical Dressings benefit. Certain dressings may be covered under other benefits.
A7 — DRESSING FOR SEVEN WOUNDS. (EFFECTIVE DATE 1/1/2003)
Surgical dressing codes billed without modifiers A1-A9 (see Coding Guidelines) are noncovered under the Surgical Dressings benefit. Certain dressings may be covered under other benefits.
A6 — DRESSING FOR SIX WOUNDS. (EFFECTIVE DATE 1/1/2003)
Surgical dressing codes billed without modifiers A1-A9 (see Coding Guidelines) are noncovered under the Surgical Dressings benefit. Certain dressings may be covered under other benefits.
A3 — DRESSING FOR THREE WOUNDS. (EFFECTIVE DATE 1/1/2003)
Surgical dressing codes billed without modifiers A1-A9 (see Coding Guidelines) are noncovered under the Surgical Dressings benefit. Certain dressings may be covered under other benefits.
A2 — DRESSING FOR TWO WOUNDS. (EFFECTIVE DATE 1/1/2003)
Surgical dressing codes billed without modifiers A1-A9 (see Coding Guidelines) are noncovered under the Surgical Dressings benefit. Certain dressings may be covered under other benefits.
KA — ADD ON OPTION/ACCESSORY FOR WHEELCHAIR (EFFECTIVE DATE 01/01/1994)
JA — ADMINISTERED INTRAVENOUSLY (EFFECTIVE DATE 01/01/2007)
N/A
JB — ADMINISTERED SUBCUTANEOUSLY (EFFECTIVE DATE 01/01/2007)
For all immune globulin (J1559, J1561, J1562, J1569) and associated infusion pump (E0779) claims where the route of administration is subcutaneous, a JB modifier must be added to each HCPCS code.
VP — APHAKIC PATIENT (EFFECTIVE DATE 01/01/1984)
N/A
TW — BACK-UP EQUIPMENT (EFFECTIVE DATE 01/01/2003)
N/A
KB — BENEFICIARY REQUESTED UPGRADE FOR ABN, MORE THAN 4 MODIFIERS IDENTIFIED ON CLAIM. (EFFECTIVE DATE 1/1/2003)
N/A
KT — BENEFICIARY RESIDES IN A COMPETITIVE BIDDING AREA AND TRAVELS OUTSIDE THAT COMPETITIVE BIDDING AREA AND RECEIVES A COMPETITIVE BID ITEM (UPDATED DATE 04/01/2008
N/A
KE — BID UNDER ROUND ONE OF THE DMEPOS COMPETITIVE BIDDING PROGRAM FOR USE WITH NON-COMPETITIVE BID BASE EQUIPMENT (EFFECTIVE 01/01/2009)
This is a pricing modifier and it used with certain items that may be used with competitive or non-competitive bid items. Use of the KE indicates the 9.5% reduction should not affect your reimbursement.
CR — CATASTROPHE/DISASTER RELATED
For use by providers that have been granted a formal waiver under §1135 of the Social Security Act and then only for services affected by the emergency and while the waiver remains in effect.
QQ — CLAIM SUBMITTED WITH A WRITTEN STATEMENT OF INTENT
CT — COMPUTED TOMOGRAPHY SERVICES FURNISHED USING EQUIPMENT THAT DOES NOT MEET EACH OF THE ATTRIBUTES OF THE NATIONAL ELECTRICAL MANUFACTURERS ASSOCIATIONS (NEMA) XR-29-2013 STANDARD
N/A
PD — DIAGNOSTIC OR RELATED NON DIAGNOSTIC ITEM OR SERVICE PROVIDED IN A WHOLLY OWNED OR OPERATED ENITY TO A PATIENT WHO IS ADMITTED AS AN INPATIENT WITHIN 3 DAYS (EFFECTIVE DATE 01/01/2012)
KL — DMEPOS ITEM DELIVERED VIA MAIL (EFFECTIVE DATE 07/01/2007)
When using this modifier, you are indicating you have delivered your supplies via mail. This modifier must only be used with diabetic supplies.
KG — DMEPOS ITEM SUBJECT TO DMEPOS COMPETITIVE BIDDING PROGRAM NUMBER 1 (EFFECTIVE DATE 07/01/2007)
KK — DMEPOS ITEM SUBJECT TO DMEPOS COMPETITIVE BIDDING PROGRAM NUMBER 2 (EFFECTIVE DATE 07/01/2007)
KU — DMEPOS ITEM SUBJECT TO DMEPOS COMPETITIVE BIDDING PROGRAM NUMBER 3 (EFFECTIVE DATE 07/01/2007)
N/A
KW — DMEPOS ITEM SUBJECT TO DMEPOS COMPETITIVE BIDDING PROGRAM NUMBER 4 (EFFECTIVE DATE 1/1/2008)
N/A
KY — DMEPOS ITEM SUBJECT TO DMEPOS COMPETITIVE BIDDING PROGRAM NUMBER 5 (EFFECTIVE DATE 1/1/2008)
N/A
KV — DMEPOS ITEM SUBJECT TO DMEPOS COMPETITIVE BIDDING PROGRAM THAT IS FURNISHED AS PART OF A PROFESSIONAL SERVICE (EFFECTIVE DATE 1/1/2008)
N/A
J4 — DMEPOS ITEM SUBJECT TO DMEPOS COMPETITIVE BIDDING PROGRAM THAT IS FURNISHED BY A HOSPITAL UPON DISCHARGE (EFFECTIVE 01/01/2010)
JW — DRUG AMOUNT DISCARDED/NOT ADMINISTERED TO ANY PATIENT (EFFECTIVE 01/01/2003)
For CGS DMEMAC claims, the JW modifier is not required for discarded drugs and biologicals.
KD — DRUG OR BIOLOGICAL INFUSED THOUGH DME. (EFFECTIVE DATE 01/01/04)
RD — DRUG PROVIDED TO BENEFICIARY, BUT NOT ADMINISTERED
EM — EMERGENCY RESERVE SUPPLY (FOR ESRD BENEFIT ONLY).
ET — EMERGENCY SERVICES
EA — ERYTHROPOETIC STIMULATING AGENT (ESA) ADMINISTERED TO TREAT ANEMIA DUE TO ANTI-CANCER CHEMOTHERAPY (EFFECTIVE DATE 1/1/2008)
EB — ERYTHROPOETIC STIMULATING AGENT (ESA) ADMINISTERED TO TREAT ANEMIA DUE TO ANTI-CANCER RADIOTHERAPY (EFFECTIVE DATE 1/1/2008)
EC — ERYTHROPOETIC STIMULATING AGENT (ESA) ADMINISTERED TO TREAT ANEMIA NOT DUE TO ANTI-CANCER RADIOTHERAPY OR ANTI-CANCER CHEMOTHERAPY (EFFECTIVE DATE 1/1/2008)
EX — EXPATRIATE BENEFICIARY
Effective July 1, 2016, use this modifier to bill Medicare for purchased only DMEPOS items that are furnished to expatriate beneficiaries. By attaching the EX modifier, the supplier is attesting that the benefidicary is an expatriate beneficiary, and that the item was delivered/furnished while the beneficiary is present in the U.S., and all other billing criteria has been met.
QA — FDA INVESTIGATIONAL DEVICE EXEMPTION (ENDS 12/31/2007)
KP — FIRST DRUG OF A MULTIPLE DRUG UNIT DOSE FORMULATION
When there is a single drug in a unit dose container, the KO modifier is added to the unit dose form code. (Exception: The KO modifier is not used with code J2545 or Q4080.) Except for code J7620, when two or more drugs are combined and dispensed to the patient in the same unit dose container, each of the drugs is billed using its unit dose form code. The KP modifier is added to only one of the unit dose form codes and the KQ modifier is added to the other unit dose code(s). Whenever a unit dose form code is billed, it must have a KO, KP or KQ modifier. (Exception: The KO, KP and KQ modifiers should not be used with code J7620.) If a unit dose code does not have one of these modifiers, it will be denied as an invalid code. The KO, KP, and KQ modifiers are not used with the concentrated form codes. The only FDA-approved unit dose preparation containing more than one drug is J7620, the combination of albuterol and ipratropium. Therefore, if the following FDA-approved unit dose codes are billed with a KP or KQ modifier, they will be rejected as invalid for claim submission: J2545, J7608, J7613, J7614, J7626, J7631, J7639, J7644, J7649, J7659, J7669, J7682, Q4080, and Q4080.
RE — FURNISHED IN FULL COMPLIANCE WITH FDA-MANDATED RISK EVALUATION AND MITIGATION STRATEGY (REMS) (EFFECTIVE 01/01/2009)
KS — GLUCOSE MONITOR SUPPLY FOR DIABETIC BENEFICIARY NOT TREATED WITH INSULIN
If the patient is not being treated with insulin injections, the KS modifier must be added to the code for the monitor and each related supply on every claim submitted.
ED — HEMATOCRIT LEVEL HAS EXCEEDED 39% (OR HEMOGLOBIN LEVEL HAS EXCEEDED 13.0 G/DL) FOR 3 OR MORE CONSECUTIVE BILLING CYCLES IMMEDIATELY PRIOR TO AND INCLUDING THE CURRENT CYCLE (EFFECTIVE DATE 1/1/2008)
EE — HEMATOCRIT LEVEL HAS NOT EXCEEDED 39% (OR HEMOGLOBIN LEVEL HAS NOT EXCEEDED 13.0 G/DL) FOR 3 OR MORE CONSECUTIVE BILLING CYCLES IMMEDIATELY PRIOR TO AND INCLUDING THE CURRENT CYCLE (EFFECTIVE DATE 1/1/2008)
Q0 — INVESTIGATIONAL CLINICAL SERVICE PROVIDED IN A CLINICAL RESEARCH STUDY THAT IS IN AN APPROVED CLINICAL RESEARCH STUDY (EFFECTIVE DATE 1/1/2008)
KF — ITEM DESIGNATED BY FDA AS CLASS III DEVICES. (EFFECTIVE DATE 04/01/04)
AV — ITEM FURNISHED IN CONJUNCTION WITH A PROSTHETIC DEVICE, PROSTHETIC OR ORTHOTIC. (EFFECTIVE DATE 1/1/2003)
AW — ITEM FURNISHED IN CONJUNCTION WITH A SURGICAL DRESSING. (EFFECTIVE DATE 1/1/2003)
AU — ITEM FURNISHED IN CONJUNCTION WITH A UROLOGICAL, OSTOMY, OR TRACHEOSTOMY SUPPLY. (EFFECTIVE DATE 1/1/2003)
This modifier is used specifically with codes A4450, A4452, and A5120.
AX — ITEM FURNISHED IN CONJUNCTION WITH DIALYSIS SERVICES. (EFFECTIVE DATE 1/1/2003)
BA — ITEM FURNISHED IN CONJUNCTION WITH PARENTERAL ENTERAL NUTRITION (PEN) SERVICES. (EFFECTIVE DATE 1/1/2003)
When an IV pole (E0776) is used in conjunction with parenteral nutrition, the BA modifier should be added to the code. Code E0776 is the only code with which the BA modifier may be used.
GZ — ITEM OR SERVICE EXPECTED TO BE DENIED AS NOT REASONABLE OR NECESSARY. (EFFECTIVE 1/1/2002)
AY — ITEM OR SERVICE FURNISHED TO AN ESRD PATIENT THAT IS NOT FOR THE TREATMENT OF ESRD (EFFECTIVE 01/01/2011)
QV — ITEM OR SERVICE PROVIDED AS ROUTINE CARE IN A MEDICARE QUALIFYING CLINICAL TRAIL (ENDS 12/31/2007)
GY — ITEM OR SERVICE STATUTORILY EXCLUDED, DOES NOT MEET THE DEFINITION OF ANY MEDICARE BENEFIT OR, FOR NON-MEDICARE INSURERS, IS NOT A CONTRACT BENEFIT (UPDATED 1/1/2008)
FB — ITEM PROVIDED W/O COST TO PROVIDER, SUPPLIER OR PRACTITIONER, OR FULL CREDIT RECEIVED FOR REPLACED DEVICE (UPDATED 1/1/2008)
QR — ITEM/SERVICE IN MEDICARE STUDY - OXYGEN (ENDS 12/31/2007)
T4 — LEFT FOOT, FIFTH DIGIT (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1830 or E1831. Failure to append the modifier will result in a rejection for incorrect coding.
T3 — LEFT FOOT, FOURTH DIGIT (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1830 or E1831. Failure to append the modifier will result in a rejection for incorrect coding.
TA — LEFT FOOT, GREAT TOE (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1830 or E1831. Failure to append the modifier will result in a rejection for incorrect coding.
T1 — LEFT FOOT, SECOND DIGIT (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1830 or E1831. Failure to append the modifier will result in a rejection for incorrect coding.
T2 — LEFT FOOT, THIRD DIGIT (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1830 or E1831. Failure to append the modifier will result in a rejection for incorrect coding.
F3 — LEFT HAND, FOURTH DIGIT (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1825. Failure to append the modifier will result in a rejection for incorrect coding.
F1 — LEFT HAND, SECOND DIGIT (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1825. Failure to append the modifier will result in a rejection for incorrect coding.
F2 — LEFT HAND, THIRD DIGIT (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1825. Failure to append the modifier will result in a rejection for incorrect coding.
FA — LEFT HAND, THUMB (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1825. Failure to append the modifier will result in a rejection for incorrect coding.
LT — LEFT SIDE. (USED TO IDENTIFY ITEM PROVIDED FOR THE LEFT SIDE OF THE BODY.)
N/A
K0 — LOWER EXTREMITY PROSTHESIS FUNCTIONAL LEVEL 0
DOES NOT HAVE THE ABILITY OR POTENTIAL TO AMBULATE OR TRANSFER SAFELY WITH OR WITHOUT ASSISTANCE AND A PROSTHESIS DOES NOT ENHANCE THEIR QUALITY OF LIFE OR MOBILITY.
K1 — LOWER EXTREMITY PROSTHESIS FUNCTIONAL LEVEL 1
HAS THE ABILITY OR POTENTIAL TO USE A PROSTHESIS FOR TRANSFERS OR AMBULATION ON LEVEL SURFACES AT FIXED CADENCE. TYPICAL OF THE LIMITED AND UNLIMITED HOUSEHOLD AMBULATOR.
K2 — LOWER EXTREMITY PROSTHESIS FUNCTIONAL LEVEL 2
HAS THE ABILITY OR POTENTIAL FOR AMBULATION WITH THE ABILITY TO TRAVERSE LOW LEVEL ENVIRONMENTAL BARRIERS SUCH AS CURBS, STAIRS OR UNEVEN SURFACES. TYPICAL OF THE LIMITED COMMUNITY AMBULATOR.
K3 — LOWER EXTREMITY PROSTHESIS FUNCTIONAL LEVEL 3
HAS THE ABILITY OR POTENTIAL FOR AMBULATION WITH VARIABLE CADENCE. TYPICAL OF THE COMMUNITY AMBULATOR WHO HAS THE ABILITY TO TRANSVERSE MOST ENVIRONMENTAL BARRIERS AND MAY HAVE VOCATIONAL, THERAPEUTIC OR EXERCISE ACTIVITY THAT DEMANDS PROSTHETIC UTILIZATION BEYOND SIMPLE LOCOMOTION.
K4 — LOWER PROSTHESIS FUNCTIONAL LEVEL 4
HAS THE ABILITY OR POTENTIAL FOR PROSTHETIC AMBULATION THAT EXCEEDS THE BASIC AMBULATION SKILLS, EXHIBITING HIGH IMPACT, STRESS, OR ENERGY LEVELS, TYPICAL OF THE PROSTHETIC DEMANDS OF THE CHILD, ACTIVE ADULT, OR ATHLETE.
SC — MEDICALLY NECESSARY SERVICE OR SUPPLY (EFFECTIVE DATE 01/01/2001)
GL — MEDICALLY UNNECESSARY UPGRADE PROVIDED INSTEAD OF NON-UPGRADED ITEM, NO CHARGE, NO ADVANCE BENEFICIARY NOTICE (ABN) (UPDATED 1/1/2008)
M2 — MEDICARE SECONDARY PAYER (MSP) (EFFECTIVE DATE 01/01/2007)
99 — MODIFIER OVERFLOW. (EFFECTIVE DATE 7/1/2003)
This modifier is used when you have exhausted the modifier field on the claim form. If you need additional room to add modifiers, append the 99 modifier to the last available field and include a narrative of other modifiers needed on the claim.
NB — NEBULIZER SYSTEM, ANY TYPE, FDA-CLEARED FOR USE WITH SPECIFIC DRUG (EFFECTIVE 01/01/2011)
NU — NEW DURABLE MEDICAL EQUIPMENT PURCHASE.
This modifier is used for new DME items that are purchased. When using the NU modifier, you are indicating you have furnished the beneficiary with a new (never used) piece of equipment.
NR — NEW WHEN RENTED
EY — NO PHYSICIAN OR OTHER LICENSED HEALTH CARE PROVIDER ORDER FOR THIS ITEM OR SERVICE. (EFFECTIVE DATE 1/1/2003)
If you do not have a prescription from the physician prior to billing Medicare, you must append the EY modifier to your claim
GX — NOTICE OF LIABILITY ISSUED, VOLUNTARY UNDER PAYER POLICY (EFFECTIVE 04/01/2010)
ZA — NOVARTIS/SANDOZ
BO — ORALLY ADMINISTERED NUTRITION, NOT BY FEEDING TUBE. (EFFECTIVE DATE 1/1/2003)
When enteral nutrients (B4149-B4162) are administered by mouth, the BO modifier must be added to the code.
QH — OXYGEN CONSERVING DEVICE IS BEING USED WITH AN OXYGEN DELIVERY SYSTEM.
FC — PARTIAL CREDIT RECEIVED FOR REPLACED DEVICE (EFFECTIVE DATE 1/1/2008)
CG — POLICY CRITERIA APPLIED (EFFECTIVE DATE 07/01/2008)
Effective for claims with dates of service on or after July 1, 2010, if an L3923 orthosis has a rigid plastic or metal component, the supplier must add the CG modifier (policy criteria applied) to the code. Claims for L3923 billed without a CG modifier will be rejected as incorrect coding. The CG modifier must be added to code L0450, L0454, L0455, L0621, L0625, or L0628 only if it is one made primarily of nonelastic material (e.g., canvas, cotton or nylon) or having a rigid posterior panel.
QF — PRESCRIBED AMOUNT OF OXYGEN EXCEEDS 4 LPM AND PORTABLE OXYGEN IS PRESCRIBED.
These modifiers may only be used with stationary gaseous (E0424) or liquid (E0439) systems or with an oxygen concentrator (E1390, E1391). They must not be used with codes for portable systems or oxygen contents.
QG — PRESCRIBED AMOUNT OF OXYGEN IS GREATER THAN 4 LITERS PER MINUTE (LPM).
These modifiers may only be used with stationary gaseous (E0424) or liquid (E0439) systems or with an oxygen concentrator (E1390, E1391). They must not be used with codes for portable systems or oxygen contents.
QE — PRESCRIBED AMOUNT OF OXYGEN IS LESS THAN 1 LITER PER MINUTE (LPM).
This modifier may only be used with stationary gaseous (E0424) or liquid (E0439) systems or with an oxygen concentrator (E1390, E1391). They must not be used with codes for portable systems or oxygen contents
CC — PROCEDURE CODE CHANGE (USE 'CC' WHEN THE PROCEDURE CODE SUBMITTED WAS CHANGED EITHER FOR ADMINISTRATIVE REASONS OR BECAUSE AN INCORRECT CODE WAS FILED). (SUPPLIERS SHOULD NOT SUBMIT MODIFIER CC.)
PL — PROGRESSIVE ADDITION LENSES (EFFECTIVE DATE 01/01/89)
GK — REASONABLE AND NECESSARY ITEM/SERVICE ASSOCIATED WITH A GA OR GZ MODIFIER (UPDATED 1/1/2008)
KR — RENTAL ITEM, BILLING FOR PARTIAL MONTH.
RP — REPLACEMENT AND REPAIR. (DELETED EFFECTIVE 12/31/2008)
RP MAY BE USED TO INDICATE REPLACEMENT OF DME, ORTHOTIC AND PROSTHETIC DEVICES, WHICH HAVE BEEN IN USE FOR SOMETIME.
RA — REPLACEMENT OF A DME ITEM (EFFECTIVE 01/01/2009)
Claims for replacement of DME items should include the RA modifer for dates of service on or after January 1, 2009.
RB — REPLACEMENT OF A PART OF DME FURNISHED AS PART OF A REPAIR (EFFECTIVE 01/01/2009)
KM — REPLACEMENT OF FACIAL PROSTHESIS INCLUDING NEW IMPRESSION/MOULAGE
KN — REPLACEMENT OF FACIAL PROSTHESIS USING PREVIOUS MASTER MODEL
KC — REPLACEMENT OF SPECIAL POWER WHEELCHAIR INTERFACE. (EFFECTIVE DATE 01/01/05)
T9 — RIGHT FOOT, FIFTH DIGIT (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1830 or E1831. Failure to append the modifier will result in a rejection for incorrect coding.
T8 — RIGHT FOOT, FOURTH DIGIT (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1830 or E1831. Failure to append the modifier will result in a rejection for incorrect coding.
T5 — RIGHT FOOT, GREAT TOE (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1830 or E1831. Failure to append the modifier will result in a rejection for incorrect coding.
T6 — RIGHT FOOT, SECOND DIGIT (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1830 or E1831. Failure to append the modifier will result in a rejection for incorrect coding.
T7 — RIGHT FOOT, THIRD DIGIT (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1830 or E1831. Failure to append the modifier will result in a rejection for incorrect coding.
F9 — RIGHT HAND, FIFTH DIGIT (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1825. Failure to append the modifier will result in a rejection for incorrect coding.
F8 — RIGHT HAND, FOURTH DIGIT (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1825. Failure to append the modifier will result in a rejection for incorrect coding.
F6 — RIGHT HAND, SECOND DIGIT (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1825. Failure to append the modifier will result in a rejection for incorrect coding.
F7 — RIGHT HAND, THIRD DIGIT (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1825. Failure to append the modifier will result in a rejection for incorrect coding.
F5 — RIGHT HAND, THUMB (EFFECTIVE DATE 01/01/1995)
Effective for dates of service on or after January 1, 2015, use with devices coded with HCPCS code E1825. Failure to append the modifier will result in a rejection for incorrect coding.
RT — RIGHT SIDE (USED TO IDENTIFY PROCEDURES PERFORMED ON THE RIGHT SIDE OF THE BODY).
Q1 — ROUTINE CLINICAL SERVICE PROVIDED IN A CLINICAL RESEARCH STUDY THAT IS IN AN APPROVED CLINICAL RESEARCH STUDY (EFFECTIVE DATE 1/1/2008)
KQ — SECOND OR SUBSEQUENT DRUG OF A MULTIPLE DRUG UNIT DOSE FORMULATION
When there is a single drug in a unit dose container, the KO modifier is added to the unit dose form code. (Exception: The KO modifier is not used with code J2545 or Q4080.) Except for code J7620, when two or more drugs are combined and dispensed to the patient in the same unit dose container, each of the drugs is billed using its unit dose form code. The KP modifier is added to only one of the unit dose form codes and the KQ modifier is added to the other unit dose code(s). Whenever a unit dose form code is billed, it must have a KO, KP or KQ modifier. (Exception: The KO, KP and KQ modifiers should not be used with code J7620.) If a unit dose code does not have one of these modifiers, it will be denied as an invalid code. The KO, KP, and KQ modifiers are not used with the concentrated form codes. The only FDA-approved unit dose preparation containing more than one drug is J7620, the combination of albuterol and ipratropium. Therefore, if the following FDA-approved unit dose codes are billed with a KP or KQ modifier, they will be rejected as invalid for claim submission: J2545, J7608, J7613, J7614, J7626, J7631, J7639, J7644, J7649, J7659, J7669, J7682, Q4080, and Q4080.
GW — SERVICE NOT RELATED TO THE HOSPICE PATIENT'S TERMINAL CONDITION. (USED FOR MEDICARE ADVANTAGE PLANS CLAIMS)
QJ — SERVICE/ITEMS PROVIDED TO A PRISONER OR PATIENT IN STATE OR LOCAL CUSTODY, HOWEVER THE STATE OR LOCAL GOVERNMENT, AS APPLICABLE, MEETS THE REQUIREMENT IN 42 CFR 411.1(B). (EFFECTIVE DATE 1/1/2003)
When there is a single drug in a unit dose container, the KO modifier is added to the unit dose form code. (Exception: The KO modifier is not used with code J2545 or Q4080.) Except for code J7620, when two or more drugs are combined and dispensed to the patient in the same unit dose container, each of the drugs is billed using its unit dose form code. The KP modifier is added to only one of the unit dose form codes and the KQ modifier is added to the other unit dose code(s). Whenever a unit dose form code is billed, it must have a KO, KP or KQ modifier. (Exception: The KO, KP and KQ modifiers should not be used with code J7620.) If a unit dose code does not have one of these modifiers, it will be denied as an invalid code. The KO, KP, and KQ modifiers are not used with the concentrated form codes. The only FDA-approved unit dose preparation containing more than one drug is J7620, the combination of albuterol and ipratropium. Therefore, if the following FDA-approved unit dose codes are billed with a KP or KQ modifier, they will be rejected as invalid for claim submission: J2545, J7608, J7613, J7614, J7626, J7631, J7639, J7644, J7649, J7659, J7669, J7682, Q4080, and Q4080.
MS — SIX MONTH MAINTENANCE AND SERVICING FEE FOR REASONABLE AND NECESSARY PARTS AND LABOR WHICH ARE NOT COVERED UNDER ANY MANUFACTURER OR SUPPLIER WARRANTY
For capped rental periods beginning prior to January 1, 2006 which have reached the 15 month rental cap, DME MACs pay claims for maintenance and servicing fees after six months have passed from the end of the final paid rental month or from the end of the period the item is no longer covered under the supplier's or manufacturer's warranty, whichever is later. A new CMN and/or physician's order is not needed for covered maintenance.
KX — SPECIFIC REQUIRED DOCUMENTATION ON FILE. (EFFECTIVE DATE 7/1/2002)
This modifier may be used to indicate that specific required documentation is on file in the patient's medical record. Documentation must be submitted upon request. Applicable policies include: Manual and power mobility bases and accessories, Glucose monitors & supplies, PAP devices and accessories, Respiratory Assist Devices (RAD), Commodes, Hospital beds and accessories, Therapeutic Shoes for Diabetics, Heavy duty walkers, Urological Supplies, Epoetin, Support surfaces - Groups 1, 2, and 3, Refractive Lenses - Anti reflective coating, tint, and oversize lenses, polycarbonate lenses, Cervical Traction devices - Codes E0849 and E0855, External infusion (insulin) pumps, High Frequency chest wall oscillation devices, Nebulizers (Brovana or Perforomist) - J7605 and J7606, Negative Pressure Wound Therapy, Patient lifts - E0636 and E1035, Speech generating devices, Wheelchair seating, Orthopedic Footwear, Home Dialysis supplies, Oral Antiemetic - J8502 and J8540.
EJ — SUBSEQUENT CLAIMS FOR A DEFINED COURSE OF THERAPY, E.G., EPO, SODIUM HYALURONATE, INFLAXIMAB.
BU — THE BENEFICIARY HAS BEEN INFORMED OF THE PURCHASE AND RENTAL OPTIONS AND AFTER 30 DAYS HAS NOT INFORMED THE SUPPLIER OF HIS/HER DECISION.
This modifier is used when you have discussed the purchase/rent option with the beneficiary, and the beneficiary has not responded within 30 days of the discussion.
BP — THE BENEFICIARY HAS BEEN INFORMED OF THE PURCHASE AND RENTAL OPTIONS AND HAS ELECTED TO PURCHASE THE ITEM.
This modifier is used when you have discussed the purchase/rent option with the beneficiary, and the beneficiary has chosen to purchase the DME item.
BR — THE BENEFICIARY HAS BEEN INFORMED OF THE PURCHASE AND RENTAL OPTIONS AND HAS ELECTED TO RENT THE ITEM.
This modifier is used when you have discussed the purchase/rent option with the beneficiary, and the beneficiary has chosen to rent the DME item.
GD — UNITS OF SERVICE EXCEEDS MEDICALLY UNLIKELY EDIT VALUE AND REPRESENTS REASONABLE AND NECESSARY SERVICES (EFFECTIVE DATE 1/1/2008)
UE — USED DURABLE MEDICAL EQUIPMENT PURCHASE.
This modifier is used for used DME items that are purchased. When using the UE modifier, you are indicating you have furnished the beneficiary with a used piece of equipment.
GU — WAIVER OF LIABILITY STATEMENT ISSUED AS REQUIRED BY PAYER POLICY, ROUTINE NOTICE (EFFECTIVE 01/01/2011)
GA — WAIVER OF LIABILITY STATEMENT ON FILE.
You must fully execute the Advanced Beneficiary Notice before appending the GA modifier to your claim. In order to have the GA modifier added to your claim after the initial determination, you must submit the ABN in paper to Written Reopenings.
dme modifier list 2016
dme procedure code list
medicare dme modifiers list
purchase modifier for dme
dme modifiers medicare
dme cpt codes
dme modifiers hcpcs
list of D
Durable Medical
what is durable medical equipment
durable equipment definition
durable equipment providers
durable medical equipment near me
dme medicare modifiers
dme modifier kx
dme modifiers 2017
dme modifiers 2016
dme purchase modifier
medicare dme modifiers 2016
medicare modifiers list
what is the kx modifier used for?
dme rental modifiers
modifier kh
dme modifiers list
dme modifier kx
dme modifiers 2016
medicare dme modifiers 2016
dme modifiers 2017
modifier bp

Ms. Pinky Maniri-Pescasio is the Founder of GoHealthcare Consulting. She is a National Speaker on Practice Reimbursement and a Physician Advocate. She has served the Medical Practice Industry for more than 25 years as a Professional Medical Practice Consultant.
search here
Archives
July 2024
March 2024
February 2024
October 2023
September 2023
August 2023
July 2023
June 2023
May 2023
April 2023
March 2023
February 2023
January 2023
November 2022
September 2022
July 2022
June 2022
May 2022
April 2022
March 2022
February 2022
October 2021
July 2021
June 2021
February 2021
January 2021
October 2020
September 2020
August 2020
July 2020
June 2020
April 2020
March 2020
December 2019
February 2019
September 2018
August 2018
February 2018
January 2018
December 2017
September 2017
August 2017
June 2017
May 2017
February 2017
October 2016
Categories
All
10 Common Reasons Claims Gets Denied And Reject
2019 New CPT Codes Medicare Payments For Virtual Services Remote Monitoring Interprofessional Consultation
Chronic-care-management-in-2017-changes
Events
In The News
Medical-modifiers
Medical-modifiers
Outsourcing Prior Authorization For Oncologic Surgery | Navigating Complexities For Improved Patient Care
Pain-management-billing
Pain-management-billing
Pain Management Billing Codes
Practice Management
Readers Question
Revenue Cycle
Spinal-fusion-billing-and-coding
Spinal-fusion-billing-and-coding
When To Use Medicare's ABN Advanced Beneficiary Notice Claim Reporting Modifiers
You Be The Biller
Your Be The Coder
BROWSE HERE
All
10 Common Reasons Claims Gets Denied And Reject
2019 New CPT Codes Medicare Payments For Virtual Services Remote Monitoring Interprofessional Consultation
Chronic-care-management-in-2017-changes
Events
In The News
Medical-modifiers
Medical-modifiers
Outsourcing Prior Authorization For Oncologic Surgery | Navigating Complexities For Improved Patient Care
Pain-management-billing
Pain-management-billing
Pain Management Billing Codes
Practice Management
Readers Question
Revenue Cycle
Spinal-fusion-billing-and-coding
Spinal-fusion-billing-and-coding
When To Use Medicare's ABN Advanced Beneficiary Notice Claim Reporting Modifiers
You Be The Biller
Your Be The Coder







 RSS Feed
RSS Feed